Adding Tirofiban on Top of Recombinant Tissue Plasminogen Activator May Improve Clinical Outcome in Acute Stroke Patients
Article information
Dear Sir:
Stroke remains the leading cause of disability-adjusted life-years and deaths in China [1]. Current guidelines recommend intravenous thrombolysis (IVT) with recombinant tissue plasminogen activator (rt-PA) for treating acute ischemic stroke (AIS) patients [2]. However, platelet activation can trigger vascular re-occlusion after IVT. Tirofiban, an antagonist for platelet glycoprotein IIb/IIIa, can prevent microvascular thrombosis and improve cerebral blood flow [3], as studies have confirmed its safety and clinical efficacy in combination with rt-PA for AIS [4,5].
Recent advancements have positioned image-guided IVT as the cornerstone of precise vascular recanalization therapy. To assess the efficacy and safety of this approach, we conducted a single-center, randomized, open-label, controlled clinical trial. The study protocol was approved by the medical ethics committee of Yantai Yuhuangding Hospital affiliated to Qingdao University (ID 2019-309). All patients provided written informed consent before enrollment. We aimed to compare the outcomes of patients receiving saline after IVT with rt-PA (rt-PA group) to those receiving intravenous tirofiban within 2–6 hours after IVT with rt-PA (rt-PA+T group). In the rt-PA+T group, tirofiban was given at the first dose of 0.4 µg/kg/min within 30 minutes, followed by continuous delivery at a rate of 0.1 µg/kg/min for 72 hours using a micropump.
Patient selection considerations included: stroke etiology was based on the Trial of ORG 10172 in Acute Stroke Treatment (TOAST), with exclusion of cardioembolism cases; and non-cardiogenic ischemic stroke patients exhibiting diffusion-weighted imaging (DWI) and fluid-attenuated inversion recovery (FLAIR) mismatch on brain magnetic resonance imaging (MRI), hospitalized within 9 hours of stroke onset at Yantai Yuhuangding Hospital between December 2019 and September 2022. Patients with acute large-vessel occlusion, and those who underwent mechanical thrombectomy after IVT, were excluded (Supplementary Methods).
A recent study demonstrated that low-dose rt-PA compared to standard-dose rt-PA reduces the incidence of symptomatic intracranial hemorrhage (sICH), making it suitable for Asians [6]. Furthermore, the Thrombolysis for Acute Wake-up and Unclear-Onset Strokes study with alteplase at 0.6 mg/kg further confirmed the safety and non-inferior efficacy of low-dose rt-PA (0.6 mg/kg) for ischemic stroke patients with unknown onset time [7].
Low-dose rt-PA (0.6 mg/kg) was chosen for the rt-PA+T group considering two factors: immediate administration of antiplatelet aggregation drugs after IVT in this group, and both previous research and safety considerations. Neurological deficits were assessed using the National Institutes of Health Stroke Scale (NIHSS; score range 0–42), and functional outcomes were evaluated using the modified Rankin Scale (mRS). The primary outcome was sICH, defined according to the European Cooperative Acute Stroke Study II criteria. Secondary outcomes included clinical efficiency and other safety measures. All outcomes were evaluated by two attending neurologists.
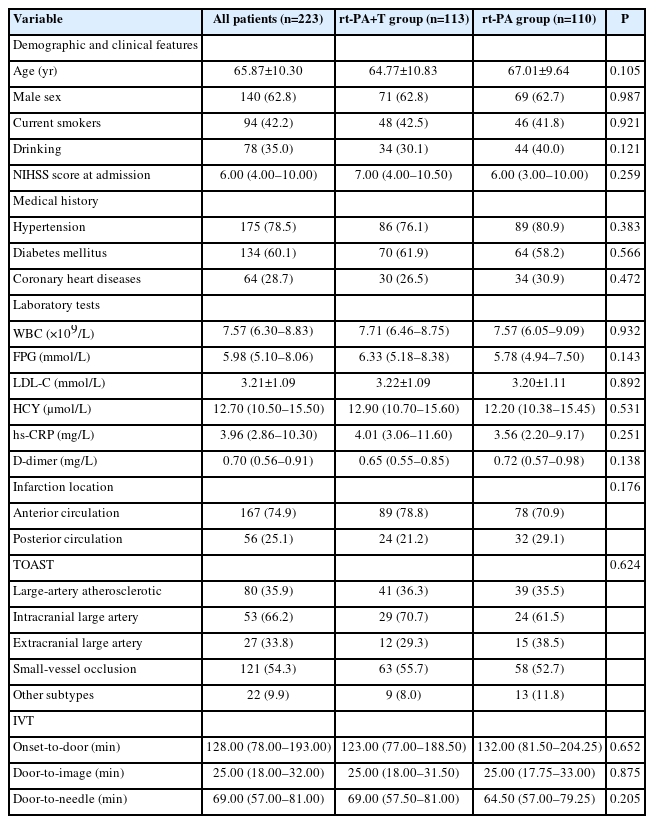
Of the 226 patients included during the study period, 3 patients in the rt-PA group were excluded due to incomplete data. Consequently, we enrolled 223 patients: 110 in the rt-PA group and 113 in the rt-PA+T group. The baseline demographic and clinical features for each group are presented in Table 1. According to the TOAST classification, 54.3% (121 patients) were diagnosed with small-vessel occlusion (SVO). The median onset-to-door time was 128 minutes (interquartile range [IQR], 78–193 min), and the median door-to-needle (DNT) time was 69 minutes (IQR, 57–81 min).
Fourteen patients in the rt-PA+T group and 16 patients in the rt-PA group developed intracranial hemorrhage (ICH) during hospitalization. Of these, five cases in the rt-PA+T group and three in the rt-PA group were classified as sICH. Statistical analysis revealed no significant differences between the two groups in the rates of sICH (4.4% vs. 2.7%, P=0.748), asymptomatic ICH (P=0.335), or bleeding from other sites (P=0.756). The 90-day all-cause mortality rate was zero in both groups. Notably, the incidence of early neurological deterioration (END; defined as a ≥4 points increase in the NIHSS score within 48 hours) was significantly lower in the rt-PA+T group compared with the rt-PA group (P=0.021) (Table 2).
The NIHSS scores at admission were similar between the groups: 7.0 (IQR, 4.0–10.5) in the rt-PA+T group and 6.0 (IQR, 3.0–10.0) in the rt-PA group (P=0.259). In both groups, NIHSS scores showed a decreasing trend over time, indicating neurological improvement. While the scores at 24 and 72 hours were not significantly different (P=0.199 and P=0.524, respectively), after 7 days, the rt-PA+T group displayed a significantly lower median NIHSS score compared to the rt-PA group (median, 3.0 vs. 4.0 [IQR, 1.0–5.0 vs. 1.0–8.0, P=0.037]), suggesting greater improvement in neurological function (Supplementary Table 1).
Additionally, the rt-PA+T group achieved a significantly higher rate of 90-day favorable prognosis (defined as 0–2 points on the mRS) compared to the rt-PA+T group (76.1% vs. 63.6%, P=0.042) (Figure 1).

Modified Rankin Scale (mRS) scores at 90 days after treatment. After 90 days, the rate of prognostic improvement (mRS 0–2) in the recombinant tissue plasminogen activator with tirofiban (rt-PA+T) group was increased compared with the recombinant tissue plasminogen activator (rt-PA) group (76.1% vs. 63.6%, P=0.042).
Our study found that the combination of tirofiban and low-dose rt-PA is a feasible and safe treatment for patients with noncardiogenic ischemic stroke within 9 hours of onset who exhibit a DWI-FLAIR mismatch on brain MRI.
Expanding the possibilities of thrombolytic therapy beyond the traditional 4.5-hour window has been a focus of clinical research, thanks to advancements in imaging analysis and the tissue window concept. However, even with IVT, successful vascular recanalization remains below 50%, and thrombolytics can trigger platelet activation, leading to vessel re-occlusion [8]. This is where tirofiban, a highly selective and reversible receptor antagonist, comes in. It inhibits platelet aggregation and potentially enhances the efficacy of IVT [9].
Building on our previous work evaluating tirofiban in ischemic stroke, we conducted a study on 60 patients within 4.5 hours of onset. Our results confirmed that adding tirofiban to IVT (rt-PA+T) significantly reduced neurological deterioration, especially in patients with SVO [5]. This finding aligns with recent studies highlighting SVO patient’s vulnerability to symptom fluctuation and deterioration [10]. Tirofiban’s ability to improve cerebral blood flow by suppressing microvascular thrombosis makes it a promising option for SVO-related stroke progression.
In our current study, over half of the participants had SVO, and the median DNT time exceeded one hour. This extended DNT might be partly attributable to the additional brain MRI requirement. Importantly, while the sICH rate was higher in the rt-PA+T group, this could be due to the inclusion of patients within 4.5 to 9 hours after stroke onset. Notably, all other safety outcomes, including sICH, asymptomatic ICH, bleeding from other sites, and mortality, showed no significant difference between the two treatment groups. This suggests that combining low-dose rt-PA with tirofiban does not increase these adverse events compared to standard-dose rt-PA alone.
Despite similar improvements in neurological function for both groups evidenced by decreasing NIHSS scores at 24 hours, 72 hours, and 7 days, the rt-PA+T group showcased significant advances. Seven days after treatment, they had a lower median NIHSS score (P=0.037) and a higher rate of favorable long-term outcomes at 90 days (P=0.042). These findings suggest that infusing tirofiban after IVT is associated with better long-term prognosis. Notably, even the rt-PA group alone achieved a 63.6% favorable prognosis rate, surpassing previous studies 60.0% [5]. This further highlights the potency of image-guided IVT as a precise revascularization therapy within the tissue window identified by DWI-FLAIR mismatch.
Our study breaks new ground by exploring ischemic stroke patients within an extended 9-hour window identified through imaging. This suggests that the DWI-FLAIR mismatch approach could expand the pool of patients benefiting from IVT’s potential. While ICH risk remains a concern, combining IVT with tirofiban appears to offer advantages including increased vascular recanalization rates, reduced END, and ultimately, improved long-term outcomes.
Limitations of this study include potential selection bias due to the use of DWI-FLAIR mismatch and the relatively small sample size, necessitating cautious interpretation of the results. Moving forward, multicenter, prospective, large-scale clinical trials are crucial to validate these findings and solidify the role of this promising approach in stroke treatment.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2023.02250.
Comparison of NIHSS scores between the two groups
Notes
Funding statement
This study was partially supported by grants from the Yantai Science and Technology Plan Project (grant numbers 2021YD033 and 2018SFGY092).
Conflicts of interest
The authors have no financial conflicts of interest.
Author contribution
Conceptualization: ZL. Study design: ZL. Methodology: WL, YZ. Data collection: LX (Liwen Xie), LJ, GZ. Investigation: RL, YH, LX (Luyao Xu), SY. Statistical analysis: RL, LX (Luyao Xu). Writing—original draft: RL. Writing—review & editing: ZL. Funding acquisition: ZL, ZS. Approval of final manuscript: all authors.
Acknowledgements
The authors thank all participants and investigators of this study for their excellent contributions.