 |
 |
- Search
| J Stroke > Volume 18(2); 2016 > Article |
|
Abstract
Background and Purpose
Recent advances in intra-arterial techniques and thrombectomy devices lead to high rate of recanalization. However, little is known regarding the effect of the evolvement of endovascular revascularization therapy (ERT) in acute basilar artery occlusion (BAO). We compared the outcome of endovascular mechanical thrombectomy (EMT) versus intra-arterial fibrinolysis (IAF)-based ERT in patients with acute BAO.
Methods
After retrospectively reviewed a registry of consecutive patients with acute ischemic stroke who underwent ERT from September 2003 to February 2015, 57 patients with acute BAO within 12 hours from stroke onset were enrolled. They were categorized as an IAF group (n=24) and EMT group (n=33) according to the primary technical option. We compared the procedural and clinical outcomes between the groups.
Results
The time from groin puncture to recanalization was significantly shorter in the EMT group than in the IAF group (48.5 [25.3 to 87.8] vs. 92 [44 to 179] minutes; P=0.02) The rate of complete recanalization was significantly higher in the EMT group than in the IAF group (87.9% vs 41.7%; P<0.01). The good outcome of the modified Rankin Scale score≤2 at 3 months was more frequent in the EMT group than in the IAF group, but it was not statistically significant (39.4% vs 16.7%; P=0.06).
Acute basilar artery occlusion (BAO) is a rare cause of stroke with a high mortality rate and an often poor clinical outcome among survivors [1,2]. Randomized trials have shown the safety and efficacy of intra-arterial fibrinolysis (IAF) given within 6 hours. Based on these trials, IAF has been used in clinical practice for many years [3-5]. However, the results of these randomized trials do not directly apply to patient with acute BAO because only few of these patients were included in the trials. Although several reports showed that IAF in acute BAO patients could improve mortality and morbidity, the rate of complete recanalization were relatively low and the mortality rate remained high [6-9]. Recent advances in intra-arterial techniques and thrombectomy devices, such as the Penumbra System (PS; Penumbra Inc., Alameda, CA, USA) and stent retrievers (Solitaire FR, Medtronic, Minneapolis, MN, USA or Trevo XP stent, Stryker, Kalamazoo, MI, USA) have led to high rates of recanalization and the stent retrievers have been proved their clinical efficacy in patients with large artery occlusion of anterior circulation [10]. However, those beneficial effects on the procedural and clinical outcomes remains unclear in patients with an acute BAO [1].
In our institution, the PS and Solitaire FR stent (Medtronic, Minneapolis, MN, USA) has been available since December, 2008 and July, 2010, respectively. We postulated and investigated that the endovascular mechanical thrombectomy (EMT)-based endovascular revascularization therapy (ERT) may improve the clinical outcome as well as procedural outcome in patients with acute BAO, compared with the traditional IAF-based ERT. Additionally, we investigated the independent predictors for good outcome 3 months after stroke onset and complete recanalization after ERT in patients with acute BAO.
This study was a retrospective one, conducted with the approval of our institutional review board, and informed consent from the patients or their legal representatives was not required.
We screened consecutive patients with acute ischemic stroke who underwent ERT at the Seoul National University Bundang Hospital from September 2003 to February 2015. A total of 614 patients were treated by ERT for acute stroke in our medical center. Clinical and radiologic data were reviewed for these patients. Clinical data included demographic information, time from stroke onset or last normal time to hospital arrival, baseline National Institutes of Health Stroke Scale (NIHSS) and modified Rankin Scale (mRS) score at 3 months. Computed tomography (CT) and/or magnetic resonance imaging (MRI) data acquired before ERT were also reviewed. All the patients underwent MRI to select patients eligible for ERT, with the exception of those patients contraindicated for MRI. For the latter, multimodal CT scans (non-contrast CT, CT angiography [CTA], and perfusion CT) were performed. Angiograms were reviewed for the location of the occlusion and reperfusion status after ERT.
Exclusion criteria were: (a) no occlusion in the basilar artery (BA; n=533), (b) >12 hours from symptom onset or first abnormal time to hospital arrival (n=13), (c) other determined etiologies like tumor invasion (n=2) and arterial dissection (n=2), (e) combined ischemic stroke of anterior circulation (n=2) or Moyamoya disease (n=1), and (f) NIHSS score≤I (n=1) or premorbid mRS ≥2 or more (n=3) (Figure 1).
All procedures were performed using the femoral artery approach and local anesthesia. A 5-Fr/6-Fr Envoy catheter or 6-Fr coaxial guiding system (Shuttle SL Flexor, Cook Medical, Bloomington, ID, USA and Envoy 5-Fr/6-Fr, Cordis, Miami Lakes, FL, USA) was introduced through a femoral sheath into the vertebra artery of concern. A heparinized saline solution was continuously perfused through the catheter during the procedure.
Generally used for treating patients with an acute BAO was multimodal combination technique consisting of (a) pharmacological thrombolysis, (b) mechanical disruption using a microguide wire or a balloon catheter, (c) permanent stenting with a balloon-expandable or self-expandable stents (Neuroform, Boston Scientific, Fremont, CA, USA; Enterprise, Codman Neurovascular, Miami Lakes, FL, USA; Solitaire, Medtronic, Minneapolis, MN, USA).
The technical strategy of the multi-modal approach of ERT has been changed at our institution due to the introduction of thrombectomy devices including the PS or Solitaire stent. Before the thrombectomy device was available, acute BAO was initially treated by suction thrombectomy using a guiding catheter to decrease the thrombus burden. Residual thrombus was then treated by pharmacological thrombolysis and mechanical disruption. If recanalization was not achieved, permanent stenting was considered for recanalization. After the PS and Solitaire stent became available, mechanical thrombectomy using either devices were initially attempted for recanalization on a case-by-case basis. The technique of forced-suction thrombectomy using a reperfusion catheter of PS without a separator was used as previously reported [11]. If mechanical thrombectomy failed, pharmacological thrombolysis, mechanical disruption or permanent stenting were considered. Therefore, we could divide patients into the IAF group and the EMT group according to the primary method for ERT.
Urokinase was used as the thrombolytic agent with a maximum dose of 800,000 U, and Glycoprotein IIb/IIIa receptor antagonist was also used (Reopro 10 mg or tirofiban 10 μg/kg as the maximum dose). Urokinase was manually infused in 20,000 U aliquots at one or two-minute intervals between doses and directly into or near the clots.
Two experienced neuroradiologists (B. S. C. and J. H. K.) who had 7 and 22 years of clinical experience, respectively, and who were unaware of the patients’ histories or treatments, scored the posterior circulation Acute Stroke Prognosis Early Computed Tomography Score (pc-ASPECTS) using the initial diffusion-weighted image (DWI) [12]. The BA was divided into three segments according to the method proposed by Archer et al. [13]. The proximal third of the BA from the vertebral artery junction to the anterior inferior cerebellar artery is the proximal segment, the middle third of the BA from the origin of the anterior inferior cerebellar artery to the origin of superior cerebellar artery is the middle segment, and the area above the origin of the superior cerebellar artery is the distal segment. Occlusion site of BA was dichotomized into caudal and mid- to proximal. Location of occlusion was defined as the site of the most inferior extension of the filling defect of the BA.
Clinical outcome measurement at 3 months after stroke onset included mortality and disability, measured according to the mRS score. We dichotomized outcome according to the functional dependency. Patients with a mRS score≤2 were in the good outcome group and those with a mRS score>2 were in the poor outcome group. The rate of complete recanalization was defined as modified treatment in cerebral infarction grade 2b or 3 [11]. The recanalization time was defined as the time interval from the puncture to the final reperfusion with modified treatment in cerebral infarction ≥2a. Procedure-related complications, such as vascular perforation or arterial dissection, were also assessed.
Symptomatic intracranial hemorrhage was defined as any hemorrhagic transformation seen on the 24-hour CT scan and associated with a decline of ≥4 points in the NIHSS score within 24 hours or leading to a patient’s death [14]. All other hemorrhages were defined as asymptomatic.
The baseline characteristics for the IAF group versus the EMT group were compared using the χ2 test for categorical variables and the Student t-test or the Mann-Whitney U test for continuous variables. For dichotomized outcome analysis, we used multivariable logistic regression analyses taking each outcome variable as a dependent variable. To obtain the cutoff DWI pc-ASPECTS for discriminating between patients with and without good outcome at 3 months regardless the complete recanalization after ERT, receiver operating characteristic curves were constructed in 53 patients who had a DWI before treatment and adjusted for the presence of complete recanalization. Significance was set at the 2-tailed P<0.05 level. We presented the values as frequencies (percentages), means (SDs), or medians (interquartile ranges), as appropriate. All statistical analyses were performed using Stata/SE 13.0 (StataCorp, College Station, TX, USA).
Among the screened 614 patients, 57 patients with an acute BAO met the inclusion and exclusion criteria for this study. Twenty-four patients were included in the IAF group and the remaining 33 patients were included in the EMT group, respectively. History of atrial fibrillation was more prevalent in the EMT group than in the IAF group (51.5% vs. 20.8%, P=0.02) Consequently, patients with cardioembolic stroke were more prevalent in the EMT group than in the IAF group, but the difference was not statistically significant (54.5% vs. 33.3%, P=0.28). In contrast to the history of atrial fibrillation, the history of hyperlipidemia was less frequent in the EMT group than in the IAF group (12.1% vs. 33.3%, P=0.05). The time from stroke onset or last normal time to hospital arrival was significantly longer in the EMT group than in the IAF group (median [interquartile ranges], 252 [114.5-451] minutes vs. 92.5 [46.3-290.8] minutes, P=0.04). The NIHSS score at initial presentation and the locations of BA occlusion were not significantly different between the groups. The baseline characteristics are summarized in Table 1.
In the IAF group, pharmacological thrombolysis was attempted in all cases. Mechanical disruption with micro-guide wire was performed in 14 (58.3%) cases. Intracranial balloon angioplasty with permanent stenting was performed in 5 (20.8%) cases. In the EMT group, 21 of 33 patients (63.6%) were treated using stent retriever, 3 were treated using Penumbra catheter, and the remaining 9 patients were treated using both devices (Supplementary Table 1). Pharmacological thrombolysis was used in 14 (42.4%) cases and the dosage of it was significantly lower in the EMT group than in the IAF group (median [interquartile ranges], 20 [20-105] U vs. 300 [170-585] U, P=0.01). The rate of mechanical disruption was significantly lower in the EMT group than in the IAF group (21.2% vs. 58.3%, P<0.01). The rate of intracranial balloon angioplasty with or without permanent stenting was relatively lower in the EMT group than in the IAF group, but it was not statistically significant (15.2% vs. 25.0%, P=0.35). The majority (83.3%) of the 6 patients who underwent intracranial balloon angioplasty in the IAF group were selected following permanent stenting, but only 2 patients (40%) of the 5 patients who underwent intracranial balloon angioplasty in the EMT group were selected following permanent stenting (P=0.09). The recanalization time was significantly shorter in the EMT group than in the IAF group (median [interquartile ranges], 48.5 [25.3 to 87.8] minutes vs. 92 [44 to 179] minutes, P=0.01). The rate of complete recanalization was significantly higher in the EMT group than in the IAF group (87.9% vs. 41.7%, P<0.01) (Table 2). In a multivariable analysis, the EMT was the only independent predictor for complete recanalization with modified treatment in cerebral infarction 2b or 3 (Table 3).
Seventeen patients with mRS≤2 at 3 months were categorized as the good outcome group in dichotomized analysis. A statistical trend that EMT group had more chance to achieve the good outcome at 3 months than IAF group was evident (39.4% vs. 12.5%, P=0.06; Figure 2 and Table 2). In a logistic regression analysis, old age, high baseline NIHSS and baseline low pc-ASPECT score were significantly associated with functional dependency at 3 months after stroke onset. When adjusting for relevant covariates, there was no factor that was significantly associated with good outcome at 3 months (Table 3). Symptomatic intracranial hemorrhage and all-cause mortality at 3 months showed no statistical differences between the groups. Procedural complications occurred in 3 cases (12.5%) of the IAF and in 7 (21.2%) of the EMT, but it was not serious.
Low baseline pc-ASPECT score based on the DWI was significantly associated with functional dependency at 3 months in a univariate model. The optimal cutoff DWI pc-ASPECTS to predict patients with favorable outcome at 3 months, regardless the presence of complete recanalization, was ≥7, with a sensitivity of 84%, specificity of 58%, and an area under the curve of 0.784 (Figure 3A). Overall, 12 (42.8%) of 28 patients with baseline ASPECT≥7 and 4 (16%) of 25 patients with baseline ASPECT<7 had good outcome at 3 months (P=0.033, Figure 3B).
EMT-based ERT for acute BAO appears to have advantages over IAF-based ERT to improve the procedural outcomes. EMT-based ERT reduced the time from groin puncture to reperfusion as well as the rates of urokinase administration and the total dose of urokinase. The rate of complete recanalization of modified treatment in cerebral infarction ≥2b in the EMT group was higher than in the IAF group (87.9% vs. 41.7%).
Several series of IAF in patients with acute BAO have been published. A meta-analysis reported a recanalization rate of 65% and mortality rate of 56% [15]. However, it is difficult to compare the results of previous studies and our series because of the diverse and ambiguous definitions of complete recanalization and different protocols of IAF in the previous studies. Additional to the IAF, several methods of mechanical clot disruption using microcatheter, microwire, balloon, or stent sought to improve recanalization rates in our series. It might facilitate pharmacologic thrombolysis by fragmenting the clot and increasing the surface area exposed to lytic agents [16,17]. Although the multimodal approaches for IAF were used, the rate of complete recanalization and good outcome were low in our series (41.7% and 16.7%, respectively). Since the era of mechanical clot extraction with advanced devices, such as the PS and stent retrievers, a high recanalization rate of 75%-100% was reported in some case series (Table 4) [18-23]. A recent study compared the procedural and clinical outcomes between the traditional IAF group and forced arterial suction thrombectomy group in acute BAO patients. They also showed the higher recanalization rate and improved clinical outcome in the forced arterial suction thrombectomy group, compared with the traditional IAF group (Table 4) [24]. However, the majority of primary devices used in the EMT group were stent retrievers contrast to the previous study. Only 3 patients were successfully recanalized by suction thrombectomy using penumbra reperfusion catheter in our study (Supplementary Table 1). Therefore, our results showed the potential advantages of stent retrievers over traditional IAF in acute BAO.
Assessment of the treatment effect of ERT in acute BAO is difficult because the natural history of basilar occlusion varies. Many confounding factors including severity at presentation, clot burden, degree of collateral flow, site of occlusion, timing of therapy and dose of thrombolytic agent could influence on the outcome [1]. At baseline, the history of atrial fibrillation was more prevalent in the EMT group than the IAF group. Although we could not know the exact cause of difference in the prevalence of atrial fibrillation between the groups, the cardioembolic etiology and the site of occlusion were not significantly associated with functional and procedural outcomes in our study (Supplementary Tables 2 and 3). In the previous literatures, there was a controversy about the relationship between the stroke subtypes and clinical outcomes in acute BAO. Distal BAO with embolic etiology was reported to be associated with poor outcome. The explanation for a poorer outcome in embolic occlusion is that emboli often lodge in the distal BA and so that these patients have less time to develop collateral circulation to maintain perfusion [2]. Controversially, a previous study reported that the occlusion in the distal one-third of the BA is associated with a lower mortality than occlusion in the proximal and/or middle portions of the BA [8]. Higher recanalization rate was reported in embolic occlusion than in atherothrombotic occlusions [8,25].
The time from symptom onset or last normal time to reperfusion was not associated with clinical outcome in our study. The time from stroke onset or last normal time to hospital arrival was longer in the EMT group than in the IAF group at baseline. It is possible that the time window for ERT has been widened due to high rate of complete recanalization with reducing recanalization time and dose of intra-arterial fibrinolytics. Although the longer time interval from onset to arrival, better procedural and clinical outcomes were achieved in the EMT group than the IAF group. There was no conclusive evidence regarding timing of therapy in patients with acute BAO. Because the patients who survive for a longer period after symptom onset may have better collateral flow, strict application of rigid temporal exclusion criteria is not warranted in BAO [1].
Application of imaging to define the extent of pretreatment core infarct will help select patients for ERT. Indeed, several studies have shown that radiologic predictors, including the brainstem DWI score or the pc-ASPECT based on CT angiography source images are independent predictors of clinical outcome in patients treated with ERT [12,26]. We measured baseline pc-ASPECT score using DWI to assess the lesion volume. The DWI-ASPECTS was reported as reliable tool for predicting poor outcome, especially in patients presenting with symptoms within 6 hours from symptom onset [27]. A score of 7 or more was predictive for good outcome at 3 months in our series. A similar result was reported in a study that investigated the predictors of good clinical outcome after stent retriever thrombectomy in acute BAO [23].
Our study has some limitations due to its retrospective design. First, the number of cases in both groups was too small to achieve statistical significance regarding the clinical outcome. Second, as the additional techniques to improve the recanalization rate were used especially in the IAF group, it was difficult to compare the efficacy of single strategies of ERT such as IAF and mechanical thrombectomy. However, this could better reflect the clinical reality. Third, as the devices used to remove the thrombus were heterogeneous in the EMT group, we could not assess the efficacy of a single device. Therefore, further studies are essential to demonstrate which thrombectomy device is more effective and safer for use in recanalization of acute BAO occlusion, e.g. the Solitaire stent or the PS. Finally, both strategies of ERT were not performed in the same time period. The IAF was a traditional endovascular treatment method. Therefore, the advances in the systemic treatment for acute ischemic stroke and the development of devices such as the guiding catheter, microcatheter, and microwire may have influenced our results.
The EMT-based ERT in acute BAO has technical advantages over IAF-based ERT in that the former reduces the time from puncture to reperfusion, and achieves more complete recanalization. These technical advantages of the EMT-based ERT could be expected to improve the clinical outcome in patients with acute BAO.
Figure 1.
Flow Sheet shows our study design and patient exclusion criteria. BAO, basilar artery occlusion; FAT, first abnormal time; LAO, large artery occlusion; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; IAF, intra-arterial fibrinolysis; EMT, endovascular mechanical thrombectomy.
Figure 2.
Modified Rankin Scale Score at 3 months. IAF, intra-arterial fibrinolysis; EMT, endovascular mechanical thrombectomy.
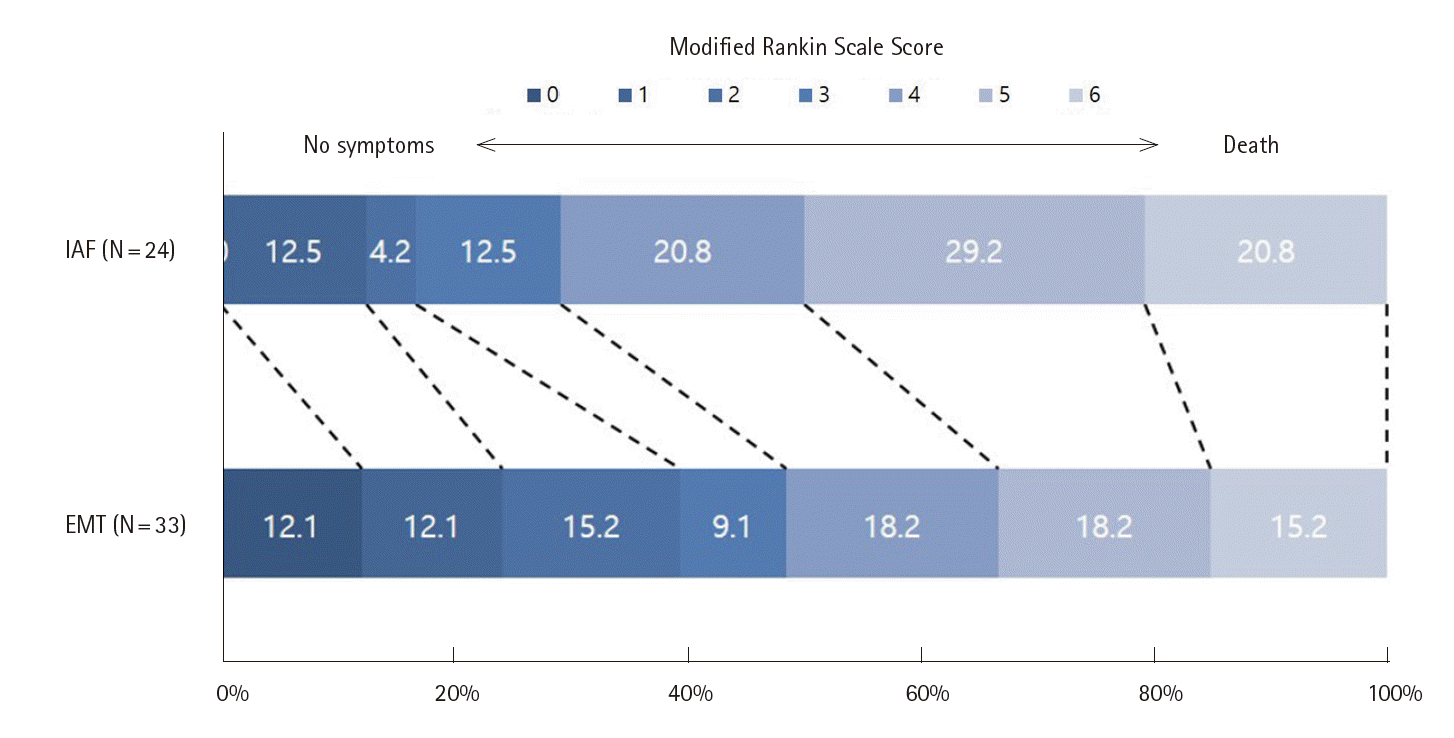
Figure 3.
Receiver operating characteristic curves of scoring of Alberta Stroke Programme Early CT Score (ASPECTS) using diffusion-weighted imaging for predicting unfavorable outcome (mRS≥3) at 3 months after adjusted for the presence of complete recanalization (A) and distribution of functional dependency at 3 months according to the baseline ASPECTS ≥7 or <7 (B).
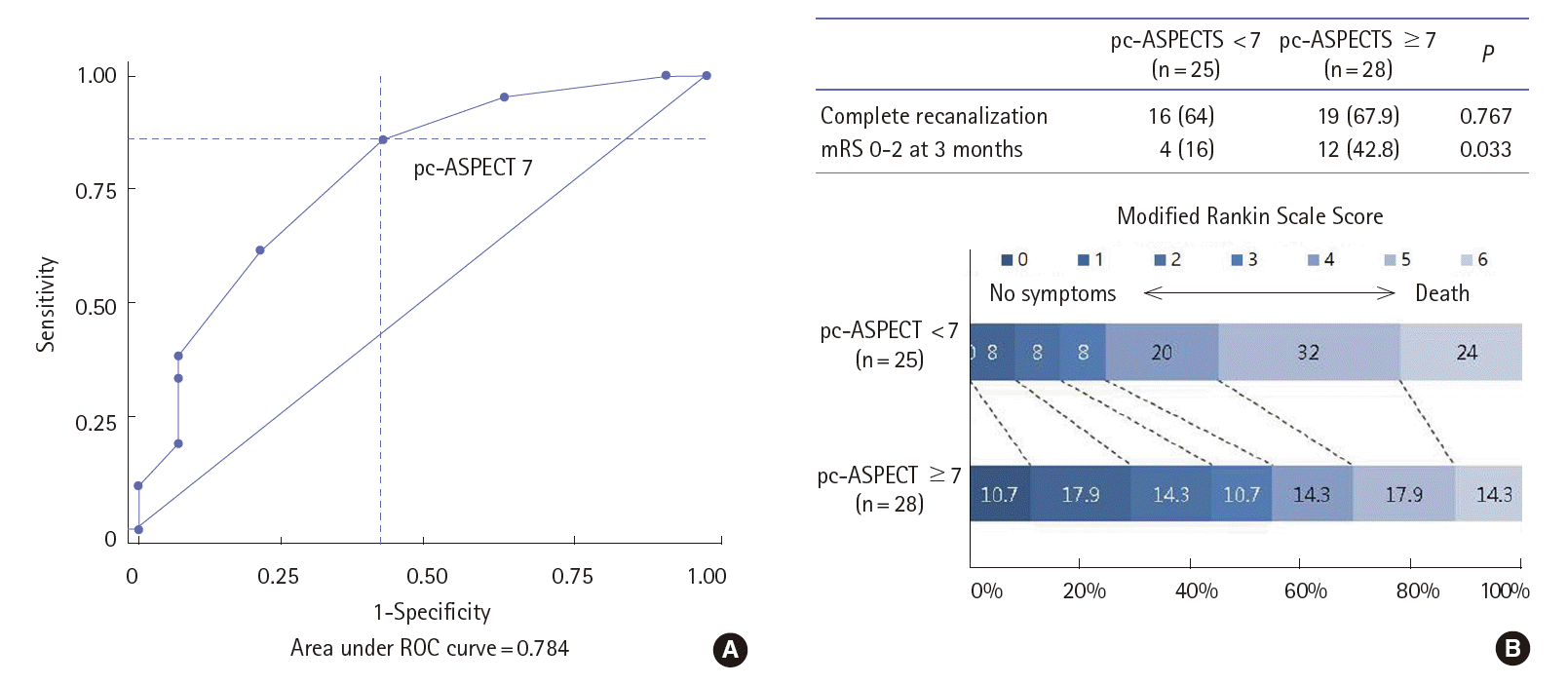
Table 1.
Baseline characteristics
IAF, intra-arterial fibrinolysis; EMT, endovascular mechanical thrombectomy; TOAST, Trial of ORG 10172 in Acute Stroke Treatment; IQR, interquartile range; LNT, Last Normal Time; NIHSS, National Institutes of Health Stroke Scale; pc-ASPECT, posterior circulation Acute Stroke Prognosis Early Computed Tomography Score; tPA, tissue Plasminogen Activator.
Table 2.
Results of endovascular revascularization therapy
|
Technical strategies of ERT |
|||
|---|---|---|---|
| IAF group (N=24) | EMT group (N=33) | P | |
| Use of intra-arterial urokinase, N (%) | 24 (100) | 14 (42.4) | < 0.01 |
| Urokinase, median [IQR] x 103, U | 300 [170-585] | 20 [20-105] | < 0.01 |
| Time from groin puncture to reperfusion*, median [IQR], (minute) | 92 [44-179] | 48.5 [25.3-87.8] | 0.01 |
| Time from stroke onset or LNT to reperfusion*, median [IQR], (minute) | 323 [221-582] | 399.5 [250.5-737.8] | 0.29 |
| Complete recanalization (mTICI ≥ 2b), N (%) | 10 (41.7) | 29 (87.9) | < 0.01 |
| Mechanical disruption, N (%) | 14 (58.3) | 7 (21.2) | < 0.01 |
| Intracranial Balloon angioplasty with or without permanent stenting, N (%) | 6 (25.0) | 5 (15.2) | 0.35 |
| Intracranial balloon angioplasty, only | 1 (4.2) | 3 (9.1) | 0.47 |
| Intracranial balloon angioplasty with permanent stenting | 5 (20.8) | 2 (6.1) | 0.09 |
| Hemorrhagic transformation | 7 (29.2) | 11 (33.3) | 0.74 |
| Symptomatic ICH, N (%) | 3 (12.5) | 2 (6.1) | 0.40 |
| Procedural complications, N (%) | 9 (37.5) | 11 (33.3) | 0.75 |
| mRS 0-2 at 90 days, N (%) | 4 (16.7) | 13 (39.4) | 0.06 |
| Mortality at 90 days, N (%) | 5 (20.8) | 5 (15.2) | 0.58 |
Table 3.
Predictors for complete recanalization with modified TICI 2b or 3 and good outcome at 3 months after stroke onset
|
Dichotomized outcome analysis |
||||
|---|---|---|---|---|
| Crude OR (95% CI) | P | Adjusted OR | P | |
| Predictors for complete recanalization* | ||||
| Strategy of ERT | ||||
| IAF | Reference | Reference | ||
| EMT | 10.15 (2.70-38.12) | < 0.01 | 23.03 (3.09-171.71) | < 0.01 |
| Predictors for good outcome at 3 months† | ||||
| Strategy of ERT | ||||
| IAF | Reference | Reference | ||
| EMT | 3.25 (0.903-11.696) | 0.07 | 2.59 (0.49-13.58) | 0.26 |
| Age, per 1 year increase | 1.08 (1.004-1.15) | 0.04 | 0.92 (0.84-1.01) | 0.09 |
| Baseline NIHSS, per 1 point increase | 1.09 (1.01-1.17) | 0.03 | 0.93 (0.84-1.03) | 0.18 |
| Baseline pc-ASPECTS, per 1 point increase | 0.66 (0.46-0.96) | 0.03 | 1.47 (0.92-2.35) | 0.10 |
TICI, treatment in cerebral infarction; OR, odds ratio; ERT, endovascular revascularization therapy; IAF, intra-arterial fibrinolysis; EMT, endovascular mechanical thrombectomy; NIHSS, National Institutes of Health Stroke Scale; pc-ASPECT, posterior circulation Acute Stroke Prognosis Early Computed Tomography Score.
Table 4.
Comparison baseline characteristics and results in previous case series and our study using mechanical thrombectomy in acute basilar artery occlusion
| References | No. of patients | Age, mean, year | Baseline NIHSS, median [IQR] | Device | Recanalization rate (%) | Good outcome of mRS 0-2 (%) | Mortality (%) |
|---|---|---|---|---|---|---|---|
| Mordasini P et al. [19] | 14 | 64.5 | 21 [5-36] | Solitaire | 100 | 29 | 36 |
| Mourand I et al. [18] | 31 | 61 | 38 [9-38] | Solitaire | 74 | 35 | 32 |
| Baek JM et al. [20] | 25 | 68 | 11 [3-25] | Solitaire | 84 | 48 | 12 |
| Möhlenbruch M et al. [21] | 24 | 70 | 24 [7-42] | Solitaire/Revive | 75 | 33 | 29 |
| Son S et al. [22] | 18 | 66.4 | 21.3 (9.7)* | Penumbra | 100 | 44.4 | 39 |
| 13 | 68.9 | 27.3 (11.0)* | Solitaire | 84.6 | 38.5 | 46 | |
| Yoon W et al. [23] | 50 | 71 | 10.5 [7.75-16] | Stent retrievers | 96 | 54 | N/A |
| Eom YI et al. [24] | 25 | 66.6 | 19.9 (8.1)* | IAF | 60 | 8† | 68 |
| 32 | 68.3 | 19.8 (10.7)* | Penumbra | 88 | 34† | 25 | |
| Our study | 24 | 70.2 | 19 [11.3-24.8] | IAF | 41.7 | 16.7 | 20.8 |
| 33 | 68.4 | 21 [12.5-28] | Stent retrievers/Penumbra | 87.9 | 39.4 | 15.2 |
References
1. Mortimer A, Bradley M, Renowden S. Endovascular therapy for acute basilar artery occlusion: a review of the literature. J Neurointerv Surg 2012;4:266-273.


2. Voetsch B, DeWitt LD, Pessin MS, Caplan LR. Basilar artery occlusive disease in the New England Medical Center Posterior Circulation Registry. Arch Neurol 2004;61:496-504.


3. Zaidat OO, Suarez JI, Santillan C, Sunshine JL, Tarr RW, Paras VH, et al. Response to intra-arterial and combined intravenous and intra-arterial thrombolytic therapy in patients with distal internal carotid artery occlusion. Stroke 2002;33:1821-1826.


4. Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA 1999;282:2003-2011.


5. Ogawa A, Mori E, Minematsu K, Taki W, Takahashi A, Nemoto S, et al. Randomized trial of intraarterial infusion of urokinase within 6 hours of middle cerebral artery stroke: the middle cerebral artery embolism local fibrinolytic intervention trial (MELT) Japan. Stroke 2007;38:2633-2639.


6. Hacke W, Zeumer H, Ferbert A, Brückmann H, del Zoppo G. Intra-arterial thrombolytic therapy improves outcome in patients with acute vertebrobasilar occlusive disease. Stroke 1988;19:1216-1222.


7. Kashiwagi J, Kiyosue H, Hori Y, Okahara M, Tanoue S, Sagara Y, et al. Endovascular recanalization of acute intracranial vertebrobasilar artery occlusion using local fibrinolysis and additional balloon angioplasty. Neuroradiology 2010;52:361-370.


8. Eckert B, Kucinski T, Pfeiffer G, Groden C, Zeumer H. Endovascular therapy of acute vertebrobasilar occlusion: early treatment onset as the most important factor. Cerebrovasc Dis 2002;14:42-50.


9. Macleod MR, Davis SM, Mitchell PJ, Gerraty RP, Fitt G, Hankey GJ, et al. Results of a multicentre, randomised controlled trial of intra-arterial urokinase in the treatment of acute posterior circulation ischaemic stroke. Cerebrovasc Dis 2005;20:12-17.


10. Song D, Cho A. Previous and recent evidence of endovascular therapy in acute ischemic stroke. Neurointervention 2015;10:51-59.



11. Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke a consensus statement. Stroke 2013;44:2650-2663.



12. Puetz V, Sylaja P, Coutts SB, Hill MD, Dzialowski I, Mueller P, et al. Extent of hypoattenuation on CT angiography source images predicts functional outcome in patients with basilar artery occlusion. Stroke 2008;39:2485-2490.


13. Archer CR, Horenstein S. Basilar artery occlusion: clinical and radiological correlation. Stroke 1977;8:383-390.


14. Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, et al. Trial design and reporting standards for intraarterial cerebral thrombolysis for acute ischemic stroke. J Vasc Interv Radiol 2003;14:S493-494.


15. Smith WS. Intra-arterial thrombolytic therapy for acute basilar occlusion: pro. Stroke 2007;38:701-703.


16. Lin R, Vora N, Zaidi S, Aleu A, Jankowitz B, Thomas A, et al. Mechanical approaches combined with intra-arterial pharmacological therapy are associated with higher recanalization rates than either intervention alone in revascularization of acute carotid terminus occlusion. Stroke 2009;40:2092-2097.


17. Sorimachi T, Fujii Y, Tsuchiya N, Nashimoto T, Harada A, Ito Y, et al. Recanalization by mechanical embolus disruption during intra-arterial thrombolysis in the carotid territory. AJNR Am J Neuroradiol 2004;25:1391-1402.


18. Mourand I, Machi P, Milhaud D, Picot MC, Lobotesis K, Arquizan C, et al. Mechanical thrombectomy with the Solitaire device in acute basilar artery occlusion. J Neurointerv Surg 2014;6:200-204.


19. Mordasini P, Brekenfeld C, Byrne J, Fischer U, Arnold M, Heldner M, et al. Technical feasibility and application of mechanical thrombectomy with the Solitaire FR Revascularization Device in acute basilar artery occlusion. AJNR Am J Neuroradiol 2013;34:159-163.



20. Baek J, Yoon W, Kim S, Jung M, Park M, Kim J, et al. Acute basilar artery occlusion: outcome of mechanical thrombectomy with Solitaire stent within 8 hours of stroke onset. AJNR Am J Neuroradiol 2014;35:989-993.



21. Möhlenbruch M, Stampfl S, Behrens L, Herweh C, Rohde S, Bendszus M, et al. Mechanical thrombectomy with stent retrievers in acute basilar artery occlusion. AJNR Am J Neuroradiol 2014;35:959-964.



22. Son S, Choi DS, Oh MK, Hong J, Kim SK, Kang H, et al. Comparison of Solitaire thrombectomy and Penumbra suction thrombectomy in patients with acute ischemic stroke caused by basilar artery occlusion. J Neurointerv Surg 2016;8:13-18.


23. Yoon W, Kim SK, Heo TW, Baek BH, Lee YY, Kang HK. Predictors of good outcome after stent-retriever thrombectomy in acute basilar artery occlusion. Stroke 2015;46:2972-2975.


24. Eom YI, Hwang YH, Hong J, Choi J, Lim Y, Kang DH, et al. Forced arterial suction thrombectomy with the penumbra reperfusion catheter in acute basilar artery occlusion: a retrospective comparison study in 2 Korean university hospitals. AJNR Am J Neuroradiol 2014;35:2354-2359.



25. Brandt T, von Kummer R, Müller-Küppers M, Hacke W. Thrombolytic therapy of acute basilar artery occlusion. Variables affecting recanalization and outcome. Stroke 1996;27:875-881.









