 |
 |
- Search
| J Stroke > Volume 20(2); 2018 > Article |
|
This article has been corrected. See "Susceptibility Vessel Sign in the ASTER Trial: Higher Recanalization Rate and More Favourable Clinical Outcome after First Line Stent Retriever Compared to Contact Aspiration" in Volume 20 on page 416.
Abstract
Background and Purpose
In the Aspiration vs. Stent Retriever for Successful Revascularization (ASTER) trial, which evaluated contact aspiration (CA) versus stent retriever (SR) use as first-line technique, the impact of the susceptibility vessel sign (SVS) on magnetic resonance imaging (MRI) was studied to determine its influence on trial results.
Methods
We included patients having undergone CA or SR for M1 or M2 occlusions, who were screened by MRI with T2* gradient recalled echo. Occlusions were classified as SVS (+) or SVS (-) in each randomization arm. Modified thrombolysis in cerebral infarction (mTICI) 2b, 2c, or 3 revascularization rates were recorded and clinical outcomes assessed by the overall distribution of modified Rankin scale (mRS) at 90 days.
Results
Among the 202 patients included, 143 patients were SVS (+) (70.8%; 95% confidence interval [CI], 64.5% to 77.1%). Overall, there was no difference in angiographic and clinical outcomes according to SVS status. However, compared to SR, CA achieved a lower mTICI 2c/3 rate in SVS (+) patients (risk ratio [RR] for CA vs. SR, 0.60; 95% CI, 0.51 to 0.71) but not in SVS (-) (RR, 1.11; 95% CI, 0.69 to 1.77; P for interaction=0.018). A significant heterogeneity in favor of superiority of first-line SR strategy in SVS (+) patients was also found regarding the overall mRS distribution (common odds ratio for CA vs. SR, 0.40 vs. 1.32; 95% CI, 0.21 to 0.74 in SVS (+) vs. 95% CI, 0.51 to 3.35 in SVS (-); P for interaction=0.038).
After the recent randomized controlled trials demonstrating the superiority of mechanical thrombectomy (MT) over standard medical management alone, attention in the field is now focused on reducing time to revascularization, optimizing imaging methods for patient selection, and evaluating the best technical approach [1-4]. The Aspiration vs. Stent Retriever for Successful Revascularization (ASTER) trial aimed to compare the efficacy and safety of first-line endovascular treatment (EVT) using the contact aspiration (CA) technique versus the standard stent retriever (SR) technique and failed to show any superiority of CA on successful reperfusion [5]. In this context, thrombus subtype may play a key role in reperfusion success [6-8]. In the ASTER trial, a majority of patients were screened with magnetic resonance imaging (MRI) for acute stroke diagnosis. Thrombus composition could therefore be studied using the susceptibility vessel sign (SVS) on gradient recalled echo (GRE) sequences [9]. Intracranial clots are SVS-positive when red blood cells (RBCs) are predominantly present within the thrombus [8,10-12]. Retrospective and monocentric studies have shown that the qualitative evaluation of SVS could help predict recanalization and functional outcome after EVT [6,13-16].
We aim to assess the impact of SVS on ASTER results with a particular focus on a potential interaction with the first-line strategy (CA vs. SR).
The trial design has been published recently [17]. In brief, the ASTER trial was a prospective, randomized, multicenter, controlled, open-label, blinded endpoint (PROBE) clinical trial. It was an academic trial designed to address the question of which first-line strategy for MT (CA or SR) obtains higher revascularization rates at the end of the endovascular procedure. Patients were recruited at eight high-volume, comprehensive stroke centers in France, all of which regularly perform both types of technique. The study, which is registered with Clinical-Trials.gov (Identifier NCT02523261), was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice. Data collection during the study is reported in Table 1. The study protocol and the consent form were approved by the Comité de Protection des Personnes Ile de France VI (ID 2015-A00830-49). The details of the trial protocol were published previously [14].
This study enrolled adults admitted with suspected ischemic stroke secondary to occlusion of the anterior circulation, within 6 hours of symptom onset. Overall inclusion and exclusion criteria are detailed in the trial protocol [17].
For the present analysis, we included patients who received EVT for middle cerebral artery (M1 or M2) occlusions on first line imaging and who were screened by MRI for the diagnosis of acute ischemic stroke. SVS was classified as present (SVS [+]) or absent (SVS [-]) according strictly to the definition of Rovira et al. [9] An independent and experienced core lab analyzed all MRIs blindly.
In line with the recommendations of the American Stroke Association and European Stroke Organization enrolled patients were given intravenous (IV) thrombolysis (if they were eligible) and transferred quickly to the catheter lab for urgent thrombectomy [2]. Patients underwent their assigned endovascular procedure (CA or SR) under general anesthesia or conscious sedation. Both techniques were conducted in accordance with good practice recommendations (minimum of three attempts before switching to another strategy; use of a proximal occlusion balloon with the SR). The CA approach was previously reported [18,19].
The primary outcome for the present ancillary study of the ASTER trial was the percentage of patients with successful revascularization defined as a modified thrombolysis in cerebral infarction (mTICI) score of 2b or 3 at the end of the assigned first-line procedure (CA vs. SR alone) [20]. Secondary angiographic efficacy outcomes included the percentage of patients with “near” complete revascularization (mTICI 2c/3) [21] and complete revascularization (mTICI 3) at the end of the assigned first-line procedure, the successful, near complete and complete revascularization at the end of all endovascular procedures, the rate of more than 2 passes of the device, and the rate of resorting to rescue therapy. The secondary efficacy clinical outcomes were global disability assessed by overall distribution of the modified Rankin scale (mRS) at 90 days (shift analysis combining scores of 5 and 6) and functional independence as defined by a 90-day mRS ≤2 [22]. Safety outcomes included intracranial hemorrhage on imaging at 24±12 hours (according to the European Cooperative Acute Stroke Study [ECASS] 3 classification) [23].
Data analysis was performed on 202 patients with isolated middle cerebral artery occlusion, having undergone MRI for the diagnosis of SVS. An unweighted Cohen κ with 95% confidence interval (CI) was calculated between readers for the presence or the absence of the SVS. Categorical variables were expressed as frequencies and percentages. Quantitative variables were expressed as mean±standard deviation, or median (interquartile range) for non-normal distribution. Normality of distributions was assessed graphically and by using the Shapiro-Wilk test.
Baseline characteristics were compared between patients with and without SVS using chi-square or Fisher exact tests for categorical variables, and Student t-test or Mann-Whitney U test for continuous variables, as appropriate. In addition, absolute standardized differences were calculated and values >20% were interpreted as meaningful differences [24].
Comparisons in angiographic and clinical outcomes between patients with and without SVS were performed after pre-specified adjustment on center, use of IV thrombolysis prior to EVT and first-line endovascular strategy using generalized linear mixed models (GLMMs) by including center as a random effect. For binary outcomes, adjusted relative risks were derived from GLMMs as effect size using a binomial distribution and a loglink function. For overall distribution of mRS (shift analysis), common odds ratio (OR) was derived from GLMM as effect size using a multinomial distribution and a cumulative logit link function. We investigated the impact of SVS on first-line strategy efficacy on angiographic and clinical outcomes by including the corresponding multiplicative interaction term in the GLMMs. All statistical tests were two-sided and P<0.05 was considered statistically significant. Data were analyzed using the SAS software package, release 9.4 (SAS Institute, Cary, NC, USA).
Between October 2015 and October 2016, a total of 381 patients were randomized in the ASTER trial. Among these, 202 patients were eligible for inclusion in the present study (Figure 1). Reasons for exclusions were no EVT (n=45), internal carotid artery occlusion (n=65), and absence or poor MRI quality prior to MT for SVS diagnosis (n=69). Of the 202 included patients, 106 patients received SR in first-line and the remaining 96 received CA. Baselines characteristics and outcomes are provided for the overall study sample (Table 1) and according to the presence or absence of SVS. For the presence or the absence of the SVS, we found an excellent agreement between readers with an unweighted Cohen κ=0.88 (95% CI, 0.81 to 0.94). SVS was diagnosed in 70.8% of cases (n=143; 95% CI, 64.5% to 77.1%) with no difference according to the site of occlusion (M1, 69.0% vs. M2, 75.4%, P=0.36). As shown in Table 1, only the rate of IV tissue plasminogen activator (t-PA) prior to MT differed between the two groups with a higher rate in SVS (+) patients compared to SVS (-) patients (71.3% vs. 50.9%, P=0.005) (Table 1).
Angiographic and main clinical outcomes are described in Table 2 according to the presence or absence of SVS. There was no difference between outcomes in patients with and without SVS. Only a non-significantly increased rate of favorable outcome in patients with SVS (+) compared to patients without was found (59.0% vs. 43.1%; adjusted risk ratio [RR], 1.27; 95% CI, 0.97 to 1.66).
When efficacy of first-line MT strategy was compared according to presence or absence of SVS, heterogeneity was found on reperfusion outcomes after the first-line strategy (Figure 2). In patients with SVS (+), successful reperfusion was less often achieved with CA than SR as first-line EVT, whereas no such difference was found in patients with SVS (-). The greater difference in treatment effect size was observed for mTICI 2c/3 rate, with an adjusted RR for CA versus SR of 0.60 (95% CI, 0.51 to 0.71) in SVS (+) patients by comparison to 1.11 (95% CI, 0.69 to 1.77) in SVS (-) (Figure 2).
The rates of number of passes >2 and use of rescue treatment were greater in patients treated with CA than SR as first-line EVT for the SVS (+) group only, with an adjusted RR of 1.61 (95% CI, 1.01 to 2.55) and 2.19 (95% CI, 1.15 to 4.14), respectively. At the end of endovascular procedure, in the SVS (+) group, first-line CA compared to first line SR remained associated with a lower mTICI 2c/3 rate (adjusted RR, 0.82; 95% CI, 0.72 to 0.92).
Regarding the overall distribution of mRS (Figure 3), a significant heterogeneity in favor of superiority of first-line SR strategy was found in patients with SVS (+). The adjusted common OR of 1 point improvement for CA versus SR (0.40 vs. 1.32; 95% CI, 0.21 to 0.74 in SVS (+) vs. 95% CI, 0.51 to 3.35 in SVS (-); P for interaction=0.038).
Similar results were found when favorable outcome was analyzed although the heterogeneity test did not reach the significance level (P=0.098). In SVS (+) patients, the favorable outcome rate was 53.3% in those treated by first-line CA and 64.4% in those treated by first-line SR (adjusted RR, 0.80; 95% CI, 0.69 to 0.92). In the SVS (-) group, the favorable outcome rate was 42.9% in patients treated by first-line CA and 43.3% in those treated by first-line SR (adjusted RR, 1.02; 95% CI, 0.67 to 1.51).
The main finding of our work is that the presence of SVS impacts the success rate of the thrombectomy strategy. Compared to CA, patients treated with SR achieve higher rates of complete reperfusion within fewer passes, which translates into a better functional outcome. In the absence of SVS, no differences were observed between the two techniques. Since the thrombus is the primary target of current reperfusion therapies, SVS evaluation on MRI before treatment appears to be relevant in order to determine the most effective MT strategy.
The SVS on a GRE sequence is defined as a hypointense signal, located at the site of the thrombus, exceeding the diameter of the contralateral artery [9]. SVS is observed in 50% to 85% of cases of hyperacute stroke, particularly in the case of a RBC-dominant thrombus, whereas a lack of SVS is indicative of a fibrin-dominant thrombus [6,7,13,14,16,25-28]. Of note, a linear variation of the RBC/fibrin ratio has been reported in samples of retrieved thrombi [29]. Hence, a cutoff ratio of erythrocyte components (>64% RBCs) has been related to successful recanalization using SR. Overall, SVS has been studied in the literature on ischemic stroke treated by IV t-PA or EVT with endpoints defined either on recanalization or functional outcome. The presence of SVS is associated with higher rates of recanalization and favourable outcome after EVT [6,7,13,15]. Conversely, the absence of SVS was related to better recanalization rate and clinical outcome after IV t-PA [14,16,30]. Interestingly, in vitro studies have shown that white thrombi, which are fibrin-rich, are not permanently embedded within the SR struts but are more prone to roll between the device and the vessel wall during retrieval [31]. On the other hand, red thrombi, are easily caught by SR [31].
We show in the present study that the rate of successful recanalization within three passes was higher in the first line SR arm than in the first line CA arm in the setting of SVS (+). Our hypothesis is that the SR struts are able to incorporate a soft RBC thrombus. This result could also be related to the use of the balloon-guiding catheter in the SR arm. Beyond the benefit of a mTICI 2b, 2c, or 3, time to reperfusion is a well-known independent prognostic factor for clinical outcome at 3 months, which underlines the importance of successful reperfusion within the first passes [1,32,33]. Additionally, numerous attempts and time consuming procedures, by increasing the risk of rethrombosis and per-procedural complications, compromise the probability of favourable clinical outcome [3,4,34]. This fact explains probably, at least in part, why in SVS (+) patients experience better clinical outcome with SR as a first line approach compared to CA.
Some limitations might be applied to our study. Since previous studies have shown that SVS (-) patients were better responders to IV t-PA than SVS (+) patients, our higher proportions of IV t-PA treatment among SVS (+) patients might be explained by a higher rate of recanalization after IV t-PA in SVS (-) patients, leading to their exclusion in the present study. This is probably why we found more patients who received IV t-PA before EVT in the SVS (+) group than in the SVS (-) group. However, our analysis was adjusted for previous IV t-PA. Although SVS identification is reliable and reproducible, with excellent interobserver and intraobserver agreements, SVS evaluation in the ASTER trial was performed on GRE sequences acquired on different MRI machines due to the multicentric design of the study. This is a significant limit, as we previously reported in an in vitro study that the accuracy of diagnosing SVS to identify RBC-dominant thrombi varies significantly among MRI machines [35]. Hence, a more reliable quantitative imaging biomarker able to overcome this limitation might be useful in order to adapt EVT strategy to the thrombus composition [7]. There is a need for biomarker comparison between different modalities including imaging and biological approaches to better define the significance of SVS. Advanced post-treatment software and acquisition sequences could be of a great interest in evaluating the composition of the thrombus, but one should keep in mind that time is still limited in this context and imaging must not delay treatment after the diagnostic confirmation. In addition, we have not been able to analyse thrombi retrieved after MT and therefore did not compare SVS and histology. However, published articles failed to find a reliable and reproducible association between the thrombi histology and recanalization results [10,36]. Indeed, there are inherent biases in assessing histologic characteristics of thrombi retrieved in vivo. IV t-PA is, when required, injected after imaging but before histological analysis and only the thrombi retrieved or parts of it are available for this analysis. Lastly, we have only included middle cerebral artery (M1 or M2) occlusions, since the skull base artefact in cases of more proximal occlusions can alter the evaluation of SVS. This certainly reduced the study sample but increased the homogeneity of the studied population.
Based on the ASTER trial population, we analysed the impact of SVS on MT strategy. With SR as a first line strategy, we found a higher rate of successful recanalization when SVS was present. Furthermore, for patients with SVS (+), and even after rescue therapy, the rate of favourable clinical outcome at 3 months was higher with SR compared to CA as the first line strategy. Thrombus MRI examination before MT thus appears relevant to adapt first line strategy in the setting of acute ischemic stroke due to large vessel occlusions. Large prospective studies using MRI clot evaluation are warranted in order to confirm this hypothesis.
Notes
Disclosure
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Mickael Mazighi reports consulting for Servier and Acticor, funding for teaching from Amgen and Pfizer, and travel reimbursements from Boehringer Ingelheim, Zeneca, and Bayer. Michel Piotin reports receipt of grants from Stryker, Medtronic, Microvention, and Balt. No other disclosures were reported.
Acknowledgments
We thank Malek Ben Maacha, MSc, clinical research associate (Department of Diagnostic and Interventional Neuroradiology, Rothschild Foundation, France) for serving as the main clinical research associate for the trial and we thank Mary Osborne-Pellegrin for her help in editing the final draft of the article. These persons did not receive specific compensation for their work related to this study.
The ASTER trial investigators are: M. Piotin, R. Blanc, H. Redjem, G. Ciccio, S. Smajda, M. Mazighi, R. Fahed, J.P. Desilles, B. Lapergue, G. Rodesch, A. Consoli, O. Coskun, F. Di Maria, F. Bourdain, J.P. Decroix, A. Wang, M. Tchikviladze, S. Evrard, F. Turjman, B. Gory, P.E. Labeyrie, R. Riva, C. Mounayer, S. Saleme, V. Costalat, A. Bonafé, O. Eker, G. Gascou, C. Dargazanli, S. Bracard, R. Tonnelet, A.L. Derelle, R. Anxionnat, H. Desal, R. Bourcier, B. Daumas-Duport, J. Berge, X. Barreau, G. Margnat, L. Djemmane, J. Labreuche, and A. Duhamel.
Figure 1.
Study flow chart. SR, stent retriever; CA, contact aspiration; ICA, internal carotid artery; MCA, middle cerebral artery; SVS, susceptibility vessel sign; MRI, magnetic resonance imaging; MT, mechanical thrombectomy.
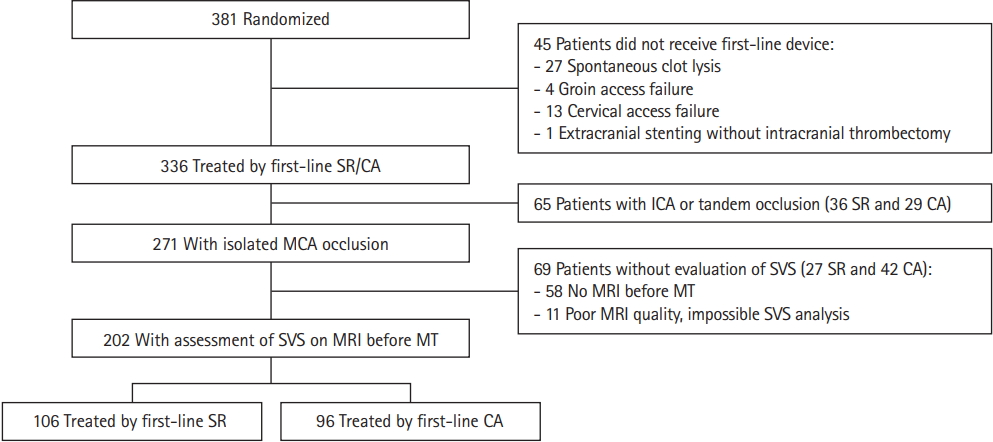
Figure 2.
Comparison in angiographic outcomes between contact aspiration (CA) versus stent retriever (SR) first-line approaches according to susceptibility vessel sign (SVS) on magnetic resonance imaging in the Aspiration vs. Stent Retriever for Successful Revascularization (ASTER) trial. Values expressed as no./total no. (%) unless otherwise indicated. Risk ratios (RRs) were calculated using first-line SR group as reference, after pre-specified adjustment for center, and intravenous thrombolysis. P Het indicates P-values for heterogeneity in treatment effect size across SVS subgroup. CI, confidence interval; mTICI, modified thrombolysis in cerebral infarction; MT, mechanical thrombectomy.
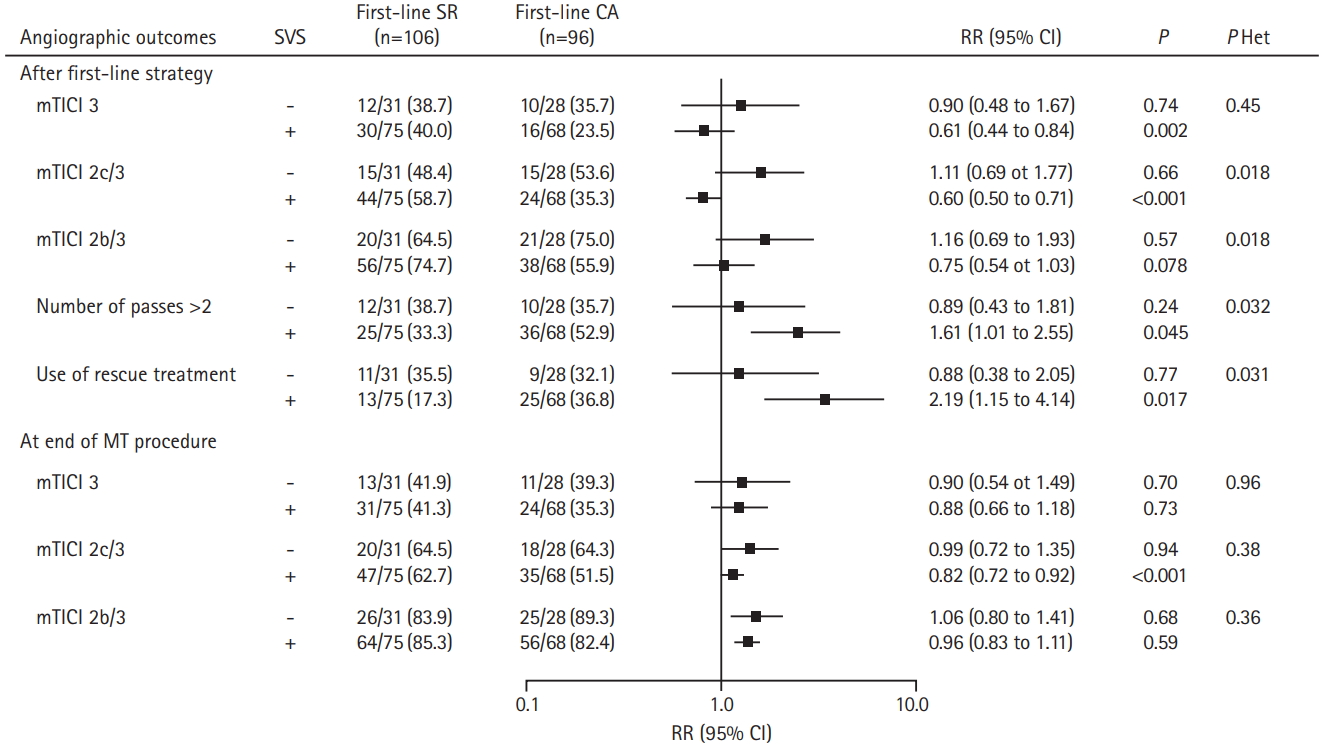
Figure 3.
Distribution of modified Rankin score (mRs) at 90 days according to first-line approaches and susceptibility vessel sign (SVS) on magnetic resonance imaging in the Aspiration vs. Stent Retriever for Successful Revascularization (ASTER) trial. SR, stent retriever; CA, contact aspiration.
Table 1.
Baseline characteristics according to susceptibility vessel sign on magnetic resonance imaging in the ASTER trial
| Characteristic | SVS (-) (n=59) | SVS (+) (n=143) | P | ASD (%) |
|---|---|---|---|---|
| Demographics | ||||
| Age (yr) | 67.6±13.7 | 67.8±16.0 | 0.94 | 1.1 |
| Men | 26/59 (44.1) | 82/143 (42.7) | 0.085 | 26.8 |
| Medical history | ||||
| Hypertension | 38/59 (64.4) | 78/139 (56.1) | 0.28 | 17.0 |
| Diabetes | 14/59 (23.7) | 21/141 (14.9) | 0.13 | 22.5 |
| Hypercholesterolemia | 21/59 (35.6) | 53/139 (38.1) | 0.74 | 5.3 |
| Current smoking | 11/51 (21.6) | 32/119 (26.9) | 0.46 | 12.5 |
| Coronary artery disease | 10/59 (17.0) | 18/138 (13.0) | 0.47 | 11.0 |
| Previous stroke or TIA | 11/58 (18.6) | 22/142 (15.5) | 0.58 | 8.4 |
| Previous antithrombotic medications | 29/58 (50.0) | 60/140 (42.9) | 0.36 | 14.4 |
| Antiplatelet | 17/58 (29.3) | 42/140 (30.0) | 0.92 | 1.5 |
| Anticoagulant | 14/58 (24.1) | 20/140 (14.3) | 0.094 | 25.2 |
| Current stroke event | ||||
| Systolic blood pressure (mm Hg) | 149±28 | 146±23 | 0.34 | 14.4 |
| NIHSS score* | 15.9±6.2 | 15.4±6.4 | 0.57 | 8.9 |
| Pre-stroke mRS ≥1 | 12/59 (20.3) | 18/143 (12.6) | 0.16 | 21.0 |
| ASPECTS* | 7 (5-8) | 7 (5-8) | 0.63 | 7.4 |
| Site of occlusion | ||||
| M1-MCA | 45/59 (76.3) | 100/143 (69.9) | 0.36 | 14.3 |
| M2-MCA | 14/59 (23.7) | 43/143 (30.1) | ||
| Favorable collaterals (ASITN 3/4) | 14/52 (26.9) | 29/126 (23.0) | 0.58 | 9.0 |
| Suspected stroke cause | ||||
| Large artery atherosclerosis | 6/59 (10.2) | 15/143 (10.5) | 1.00 | 1.3 |
| Cardioembolic | 23/59 (39.0) | 55/143 (38.5) | ||
| Other or unknown | 30/59 (50.9) | 73/143 (51.1) | ||
| Intravenous rt-PA | 30/59 (50.9) | 102/143 (71.3) | 0.005 | 43.0 |
| Endovascular treatment | ||||
| First-line CA strategy | 28/59 (47.5) | 68/143 (47.6) | 0.99 | 0.0 |
| General anesthesia | 10/59 (17.0) | 15/143 (10.5) | 0.20 | 18.9 |
| Onset to groin puncture time (min)† | 235 (159-295) | 220 (180-270) | 0.36 | 13.9 |
| Onset to imaging† | 108 (72-154) | 109 (82-143) | 0.70 | 5.9 |
| Imaging to groin puncture | 1115 (65-151) | 106 (63-142) | 0.25 | 17.6 |
Values are presented as mean±standard deviation, number/total number (%), or median (interquartile range).
ASTER, Aspiration vs. Stent Retriever for Successful Revascularization; SVS, susceptibility vessel sign; ASD, absolute standardized difference; TIA, transient ischemic attack; NIHSS, National Institutes of Health Stroke Scale; mRS, modified Rankin scale; ASPECTS, Alberta Stroke Program Early Computed Tomography Score; MCA, middle cerebral artery; ASITN, American Society of Interventional and Therapeutic Neuroradiology; rt-PA, recombinant tissue plasminogen activator; CA, contact aspiration.
Table 2.
Comparison in clinical and angiographic efficacy outcomes according to susceptibility vessel sign on magnetic resonance imaging in the ASTER trial
| Outcome | SVS (-) (n=59) | SVS (+) (n=143) | RR (95% CI)* | P* |
|---|---|---|---|---|
| Angiographic outcomes | ||||
| Reperfusion after first-line strategy | ||||
| mTICI 3 | 22/59 (37.3) | 46/143 (32.2) | 0.91 (0.76-1.10) | 0.31 |
| mTICI 2c/3 | 30/59 (50.9) | 68/143 (47.6) | 0.91 (0.73-1.12) | 0.36 |
| mTICI 2b/3† | 41/59 (69.5) | 94/143 (65.7) | 0.81 (0.53-1.25) | 0.35 |
| Number of passes >2 | 22/59 (37.3) | 61/143 (42.7) | 1.06 (0.76-1.47) | 0.73 |
| Use of rescue therapy | 20/59 (33.9) | 38/143 (26.6) | 0.89 (0.71-1.11) | 0.31 |
| Reperfusion at end of procedure | ||||
| mTICI 3 | 24/59 (40.7) | 55/143 (38.5) | 0.97 (0.80-1.19) | 0.80 |
| mTICI 2c/3 | 38/59 (64.4) | 82/143 (57.3) | 0.84 (0.63-1.13) | 0.26 |
| mTICI 2b/3 | 51/59 (86.4) | 120/143 (83.9) | 0.80 (0.41-1.54) | 0.50 |
| Procedural complications | 11/59 (18.6) | 27/143 (18.9) | 0.97 (0.88-1.07) | 0.53 |
| Clinical outcomes | ||||
| Functional independence at 3 months | 25/58 (43.1) | 82/139 (59.0) | 1.27 (0.97-1.66) | 0.079 |
| Modified Rankin scale at 3 months | 3 (1-5) | 2 (1-4) | 1.52 (0.86-2.68)‡ | 0.15 |
| ICH at 24 hours | 25/59 (42.4) | 60/140 (42.9) | 0.94 (0.75-1.15) | 0.53 |
| Symptomatic ICH | 1/59 (1.7) | 7/140 (5.0) | NE | 0.44 |
Values are presented as number/total number (%) or median (interquartile range).
ASTER, Aspiration vs. Stent Retriever for Successful Revascularization; SVS, susceptibility vessel sign; RR, risk ratio; CI, confidence interval; mTICI, modified thrombolysis in cerebral infarction; ICH, intracranial hemorrhage; NE, not estimable.
References
1. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723-1731.


2. Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, et al. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart association/American Stroke Association. Stroke 2015;46:3020-3035.


3. Yoo AJ, Andersson T. Thrombectomy in acute ischemic stroke: challenges to procedural success. J Stroke 2017;19:121-130.




5. Lapergue B, Blanc R, Gory B, Labreuche J, Duhamel A, Marnat G, et al. Effect of endovascular contact aspiration vs stent retriever on revascularization in patients with acute ischemic stroke and large vessel occlusion: the ASTER randomized clinical trial. JAMA 2017;318:443-452.



6. Bourcier R, Volpi S, Guyomarch B, Daumas-Duport B, Lintia-Gaultier A, Papagiannaki C, et al. Susceptibility vessel sign on MRI predicts favorable clinical outcome in patients with anterior circulation acute stroke treated with mechanical thrombectomy. AJNR Am J Neuroradiol 2015;36:2346-2353.



7. Bourcier R, Brecheteau N, Costalat V, Daumas-Duport B, Guyomarch-Delasalle B, Desal H, et al. MRI quantitative T2* mapping on thrombus to predict recanalization after endovascular treatment for acute anterior ischemic stroke. J Neuroradiol 2017;44:241-246.


8. Liebeskind DS, Sanossian N, Yong WH, Starkman S, Tsang MP, Moya AL, et al. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke 2011;42:1237-1243.



9. Rovira A, Orellana P, Alvarez-Sabín J, Arenillas JF, Aymerich X, Grivé E, et al. Hyperacute ischemic stroke: middle cerebral artery susceptibility sign at echo-planar gradient-echo MR imaging. Radiology 2004;232:466-473.


10. Kim J, Park JE, Nahrendorf M, Kim DE. Direct thrombus imaging in stroke. J Stroke 2016;18:286-296.




11. Park MG, Oh SJ, Baik SK, Jung DS, Park KP. Susceptibility-weighted imaging for detection of thrombus in acute cardioembolic stroke. J Stroke 2016;18:73-79.




12. Kim BJ, Kang HG, Kim HJ, Ahn SH, Kim NY, Warach S, et al. Magnetic resonance imaging in acute ischemic stroke treatment. J Stroke 2014;16:131-145.



13. Soize S, Batista AL, Rodriguez Regent C, Trystram D, Tisserand M, Turc G, et al. Susceptibility vessel sign on T2* magnetic resonance imaging and recanalization results of mechanical thrombectomy with stent retrievers: a multicentre cohort study. Eur J Neurol 2015;22:967-972.


14. Kimura K, Sakamoto Y, Iguchi Y, Shibazaki K. Clinical and MRI scale to predict very poor outcome in tissue plasminogen activator patients. Eur Neurol 2011;65:291-295.


15. Kim SK, Yoon W, Heo TW, Park MS, Kang HK. Negative susceptibility vessel sign and underlying intracranial atherosclerotic stenosis in acute middle cerebral artery occlusion. AJNR Am J Neuroradiol 2015;36:1266-1271.



16. Aoki J, Kimura K, Shibazaki K, Sakamoto Y, Saji N, Uemura J. Location of the susceptibility vessel sign on T2*-weighted MRI and early recanalization within 1 hour after tissue plasminogen activator administration. Cerebrovasc Dis Extra 2013;3:111-120.



17. Lapergue B, Labreuche J, Blanc R, Barreau X, Berge J, Consoli A, et al. First-line use of contact aspiration for thrombectomy versus a stent retriever for recanalization in acute cerebral infarction: the randomized ASTER study protocol. Int J Stroke 2018;13:87-95.


18. Turk AS, Frei D, Fiorella D, Mocco J, Baxter B, Siddiqui A, et al. ADAPT FAST study: a direct aspiration first pass technique for acute stroke thrombectomy. J Neurointerv Surg 2014;6:260-264.


19. Kowoll A, Weber A, Mpotsaris A, Behme D, Weber W. Direct aspiration first pass technique for the treatment of acute ischemic stroke: initial experience at a European stroke center. J Neurointerv Surg 2016;8:230-234.


20. Tomsick T, Broderick J, Carrozella J, Khatri P, Hill M, Palesch Y, et al. Revascularization results in the Interventional Management of Stroke II trial. AJNR Am J Neuroradiol 2008;29:582-587.



21. Almekhlafi MA, Mishra S, Desai JA, Nambiar V, Volny O, Goel A, et al. Not all "successful" angiographic reperfusion patients are an equal validation of a modified TICI scoring system. Interv Neuroradiol 2014;20:21-27.



22. Savitz SI, Lew R, Bluhmki E, Hacke W, Fisher M. Shift analysis versus dichotomization of the modified Rankin scale outcome scores in the NINDS and ECASS-II trials. Stroke 2007;38:3205-3212.


23. Hacke W, Donnan G, Fieschi C, Kaste M, von Kummer R, Broderick JP, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004;363:768-774.


25. Bae YJ, Jung C, Kim JH, Choi BS, Kim E, Han MK, et al. Potential for the use of the Solitaire stent for recanalization of middle cerebral artery occlusion without a susceptibility vessel sign. AJNR Am J Neuroradiol 2014;35:149-155.



26. Vanaerde O, Budzik JF, Mackowiak A, Norberciak L, Uettwiller M, Leclerc X, et al. Comparison between enhanced susceptibility-weighted angiography and time of flight sequences in the detection of arterial occlusion in acute ischemic stroke. J Neuroradiol 2017;44:210-216.


27. Kang DW, Jeong HG, Kim DY, Yang W, Lee SH. Prediction of stroke subtype and recanalization using susceptibility vessel sign on susceptibility-weighted magnetic resonance imaging. Stroke 2017;48:1554-1559.



28. Zhang R, Zhou Y, Liu C, Zhang M, Yan S, Liebeskind DS, et al. Overestimation of susceptibility vessel sign: a predictive marker of stroke cause. Stroke 2017;48:1993-1996.


29. Hashimoto T, Hayakawa M, Funatsu N, Yamagami H, Satow T, Takahashi JC, et al. Histopathologic analysis of retrieved thrombi associated with successful reperfusion after acute stroke thrombectomy. Stroke 2016;47:3035-3037.


30. Seners P, Turc G, Maïer B, Mas JL, Oppenheim C, Baron JC. Incidence and predictors of early recanalization after intravenous thrombolysis: a systematic review and meta-analysis. Stroke 2016;47:2409-2412.


31. Machi P, Jourdan F, Ambard D, Reynaud C, Lobotesis K, Sanchez M, et al. Experimental evaluation of stent retrievers’ mechanical properties and effectiveness. J Neurointerv Surg 2017;9:257-263.


32. Angermaier A, Michel P, Khaw AV, Kirsch M, Kessler C, Langner S. Intravenous thrombolysis and passes of thrombectomy as predictors for endovascular revascularization in ischemic stroke. J Stroke Cerebrovasc Dis 2016;25:2488-2495.


33. Costalat V, Machi P, Lobotesis K, Maldonado I, Vendrell JF, Riquelme C, et al. Rescue, combined, and stand-alone thrombectomy in the management of large vessel occlusion stroke using the solitaire device: a prospective 50-patient single-center study: timing, safety, and efficacy. Stroke 2011;42:1929-1935.


34. Balasubramaian A, Mitchell P, Dowling R, Yan B. Evolution of endovascular therapy in acute stroke: implications of device development. J Stroke 2015;17:127-137.










