Introduction
There are apparent differences in responsiveness to intravenous thrombolysis (IVT) and anticoagulation between Asians, or more strictly speaking Japanese, and other populations [1,2]. For example, the officially approved dosage of intravenous alteplase in IVT for stroke is 0.6 mg/kg in Japan versus 0.9 mg/kg in other countries according to the results of a domestic trial [3,4]. An international normalized ratio (INR) of prothrombin time of 1.6 to 2.6 is officially recommended for stroke patients aged ≥70 years with non-valvular atrial fibrillation (NVAF) in Japan [4,5]; this target range, which is lower than the global recommendation, was also found to be safe and effective as primary prevention for thromboembolic events [6]. Furthermore, the Japanese official dosage of rivaroxaban for NVAF patients [7], as well as that of prasugrel for patients with coronary artery disease [8], is also lower than the official Western dosage. The differences in dosing regimens are partly due to a higher risk of intracranial hemorrhage (ICH) as a complication and partly due to different metabolic activity for anticoagulants [1,2,9-11]. Thus, distinctive strategies for IVT specific to Japanese patients are necessary, in particular guidelines for IVT during anticoagulant medication.
Previous strategies for anticoagulated patients in the initial (2005) and second (2012) editions of the Japanese guidelines on stroke thrombolysis
Intravenous alteplase was officially approved for clinical use in hyperacute ischemic stroke in 2005 on the basis of the results of the Japan Alteplase Clinical Trial (J-ACT) with a dosage of 0.6 mg/kg in Japan [3], 9 years after approval in the United States. The Japan Stroke Society published the “Guidelines for intravenous application of rt-PA (alteplase), October 2005” [12] and organized training sessions for proper use of recombinant tissue-type plasminogen activator (rt-PA) using these guidelines as the standard all over Japan in an effort to promote the safe implementation of this therapy nationwide. Concern about a higher risk of post-IVT ICH partly led to both a long delay in regulatory approval and a distinctive low-dosage of alteplase in clinical use. Alteplase at a dose of 0.6 mg/kg has an identical intensity as duteplase at a dose of 20 mega unit (MU)/kg for patients weighing 60 kg, an optimal regimen in a domestic dose-escalation trial for hyperacute stroke [15]. The optimal dosage of intravenous alteplase for acute coronary syndrome in studies from Japan was around three-fifths of that from Western countries [16]. The lower-dosage IVT was proven to be feasible for Japanese patients in post-marketing surveillance and registry studies [17-19] and for non-Japanese populations in the recent ENhanced Control of Hypertension and Thrombolysis strokE stuDy (ENCHANTED) trial [20]. In the initial edition of the Japanese special guidelines on IVT, IVT was contraindicated for stroke patients taking warfarin with INR >1.7 and those receiving heparin with activated partial thromboplastin time (aPTT) >1.5 times the baseline value. These cut-off values of anticoagulant markers followed guidelines in other countries, since patients with higher values were excluded from the J-ACT, and their safety remained unknown [12]. Findings from the Get With The Guidelines-Stroke Registry study supported the validity of the cut-off INR value of 1.7 [21].
The second edition of the Japanese guidelines on IVT was published in 2012 concurrently with extended insurance coverage of the therapeutic time window from 3 to 4.5 hours from symptom onset [13]. At the time, reports on IVT for direct oral anticoagulant (DOAC) users had been few. Expert opinions were negative towards IVT for DOAC users within 48 hours after the last dosing or unless any relevant anticoagulant activity was ruled out by thrombin time, ecarin clotting time, or the Hemoclot test if taking dabigatran and the anti-Xa activity test if taking factor Xa inhibitors [22]. These anticoagulation tests are time-consuming or not available in regular clinical use and inappropriate for recommending as routine practice in the guidelines. Another review mentioned that IVT could be considered, for example, if 12 hours had passed from the last dosing of dabigatran, aPTT is normal, and the results of other specific anticoagulation tests are only slightly elevated, and if 6 hours had passed from the last dosing of rivaroxaban or apixaban with normal levels of aPTT and prothrombin time [23]. Since alteplase had already been limited to a lower dosage in Japan than the global recommendation, the committee members of the second edition did not want to add excessively strict limitations to IVT for anticoagulated patients. The members tried to determine eligibility for IVT based on clinically-used anticoagulation tests and time from the last dosing, as in the above review. Thus, the cut-off levels of anticoagulation parameters were taken from the initial edition, so that patients should be considered ineligible for IVT if they have an INR of >1.7 or an aPTT of >1.5 times the baseline value, even when patients taking DOACs. With respect to the cut-off of the dosing-to-needle time, opinions varied among the members from no limits to 12 hours. The members finally agreed not to limit the dosing-toneedle time in the headline recommendation, but they noted in the main text that special care should be taken in determining whether the potential benefits of IVT outweigh the potential risks in patients on regular DOAC medication until approximately half a day after the time of the last dose, because DOACs have a half-life of around 12 hours, and the eligibility criteria for IVT in DOAC users will be modified based on future research outcomes. In addition, another headline recommendation noted that, for any patients on antithrombotic therapy, especially on oral anticoagulant therapy, the decision-making for IVT should be determined with care.
A full description of the previous recommendations in the second edition is shown in Table 1.
Follow-up surveys after the second edition
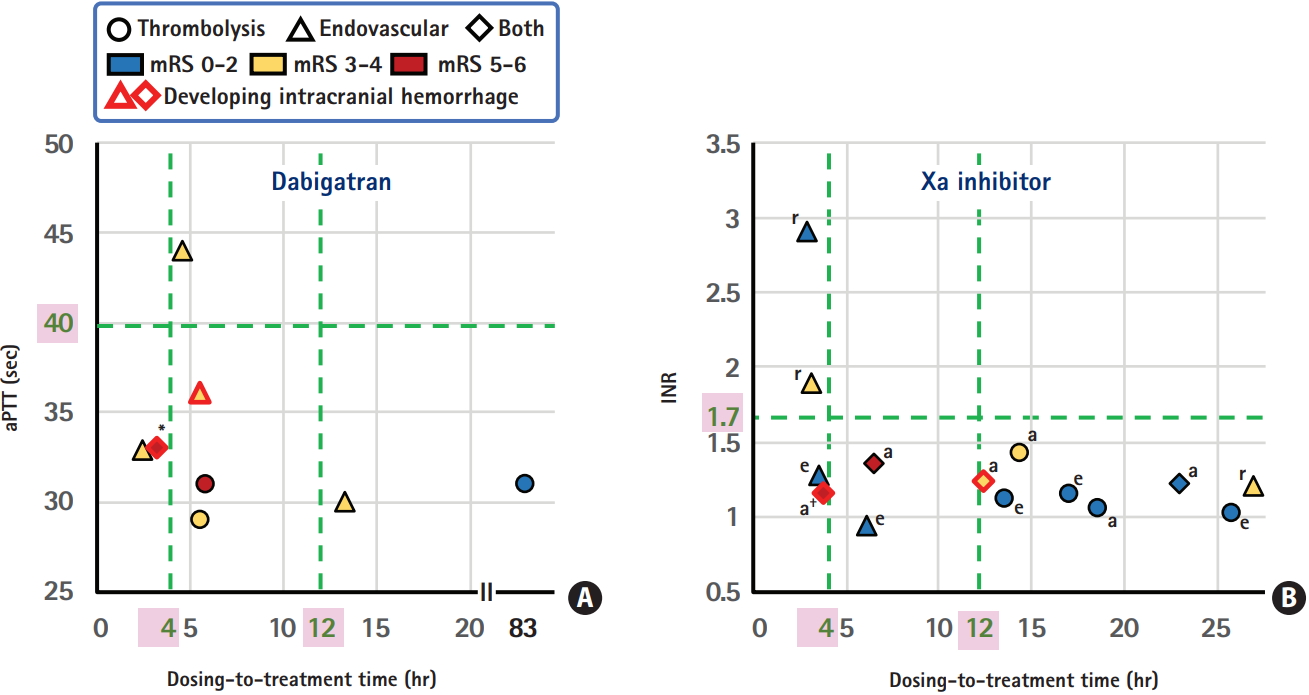
From our single-center National Cerebral and Cardiovascular Center (NCVC) Stroke Registry [24-26], a total of 22 patients (three women, median 75 years old) who visited our emergency room within 4.5 hours after symptom onset and received IVT or acute endovascular therapy, including percutaneous transluminal angioplasty in one patient and mechanical thrombectomy in the others, were studied (unpublished data). Table 2 shows the demographic characteristics, therapeutic process, and outcomes of these patients. Figure 1 shows the relationship between the time from the last dose of DOACs to initiation of IVT (or arterial puncture for patients undergoing direct endovascular therapy: dosing-to-treatment time), levels of aPTT or INR, and clinical outcomes. In our institute, aPTT is measured using the Actin FSL (Siemens Healthcare Diagnostics Inc., Tarrytown, NY, USA); mean aPTT in non-anticoagulated patients is approximately 27 to 28 seconds, and 40 seconds is roughly regarded as 1.5 times the baseline value. Nine patients received direct endovascular therapy by skipping IVT (triangular markers in the figure); three of them showed higher levels of aPTT or INR than the recommended cut-off levels, two skipped IVT due to a recent history of surgery, and the other four skipped IVT mainly because the dosing-to-treatment time was approximately <6 hours and close to the expected peak concentration time of DOACs.
ICH occurred in four patients (18%, markers with red rims). One patient developed a symptomatic subarachnoid hemorrhage, and the other three did not show any neurological deterioration. One patient developed ICH after direct thrombectomy, and the other three developed ICH after IVT plus thrombectomy; the thrombolysis in cerebral infarction scale after thrombectomy was 0 in the patient with symptomatic subarachnoid hemorrhages. The dosing-to-treatment time in the four ICH patients varied between 3.3 and 12.8 hours. When the patients were divided in half depending on the median 6 hours for the dosing-to-treatment time, three patients (27%) in the shorter-time group versus one (9%) in the longer-time group had ICH. Nine patients with a modified Rankin Scale (mRS) score of 0 to 2 at 3 months had wide variation of the dosing-to-treatment time, between 3.6 and 83.3 hours (median 17.1 hours). On the other hand, four patients with a mRS score of 5 to 6 at 3 months had relatively narrow variation between 3.3 and 6.5 hours (median 4.8 hours).
A multicenter survey involving 118 stroke centers was performed in 2015, including 100 patients (44 women, 76±10 years old) who developed ischemic stroke while taking DOACs and received acute reperfusion therapy [27]. The patients did not overlap with our single-center cases. Of these, 56 patients received IVT alone, 29 received endovascular therapy alone, and the other 15 received both. Twenty patients (20%) developed ICH within 24 hours after the therapy; in two patients it was symptomatic, defined as a ≥4-point increase of the National Institutes of Health Stroke Scale (NIHSS) score, and both patients received endovascular therapy alone. The dosing-to-treatment time was available in 52 patients; of these, five of 13 patients (38%) with a dosingto-treatment time ≤4 hours and four of 39 (10%) with a dosingto-treatment time >4 hours had ICH (P=0.033).
Major points derived from the two surveys are: (1) approximately 20% frequency of post-treatment ICH; (2) relatively higher percentage of ICH in the group with dosing-to-treatment time within 4 to 6 hours; (3) absence of symptomatic ICH after IVT alone; and (4) concentration of patients with death or bedridden state at 3 months in those with dosing-to-treatment time within 6.5 hours, although the poor outcome was not caused by post-treatment ICH in most of them. The last finding may be by chance. The two surveys indicated that IVT for DOAC users according to the second edition of the Japanese guidelines was generally safe, but IVT within 4 to 6 hours after the last dosing might be associated with excess risk of asymptomatic ICH.
A new strategy for acute reperfusion therapy following administration of idarucizumab
Idarucizumab is a humanized monoclonal antibody fragment that binds dabigatran with high affinity and specificity and rapidly reverses its anticoagulant activity [28]. On the other hand, idarucizumab does not bind other coagulant factors, does not refill coagulant factors, and has no effects on prothrombin activating ability, platelet aggregation, or endogenous thrombin generation [28,29]. Thus, it has been thought to have no influence on the coagulation cascade. The agent is in worldwide use based on the results of an interim or final analysis of the Reversal Effects of Idarucizumab on Active Dabigatran (RE-VERSE AD) study [30,31]. Idarucizumab has been in clinical use since November 2016 in Japan.
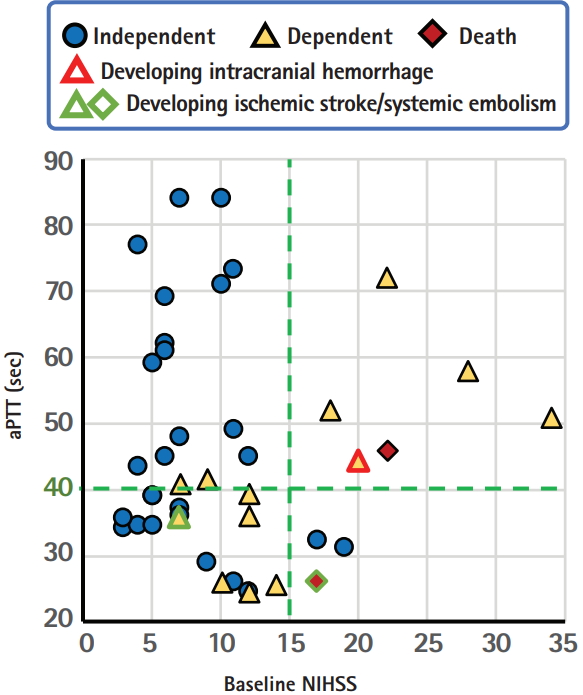
The specific and rapid effect of idarucizumab as an antidote for dabigatran without an effect on the coagulation cascade may permit us to reverse the activity of dabigatran in patients with hyperacute stroke and then to perform IVT. A total of 47 cases following this new strategy were collected without overlap from 21 articles that were published in English up to February 2018 and could be identified using PubMed with the search terms ‘stroke’ and ‘idarucizumab.’ [32-52] One case in our institute was added. Table 3 shows the background characteristics and clinical courses of the 48 cases (23 women, median 76.5 years old). Two patients developed ICH, two developed recurrent ischemic stroke, and another one developed deep vein thrombosis and bilateral pulmonary embolism; all of them died or were bedridden. Figure 2 shows the relationships between the baseline NIHSS score, levels of aPTT, and outcomes in 42 patients whose data for baseline NIHSS scores were available. Clinical outcomes were assessed mainly at hospital discharge, and independency was identified mainly based on the mRS score (score 0 to 2 was defined as independent) and sometimes estimated based on case description. Interestingly, an aPTT of 40 seconds is likely a good cut-off level to predict outcomes. Two patients with recurrent ischemic events had baseline aPTT levels <40 seconds (25.9 and 35.5 seconds). The proportion of independent patients was 88% (14/16) if aPTT >40 seconds and 65% (11/17) if aPTT <40 seconds when limited to patients with mild or moderate initial neurological severity with the NIHSS score <15 (P=0.12 by the chisquare test); this might suggest the potential enhancement of prothrombic state by idarucizumb in cases with normal or subnormal aPTT levels. We should note that this analysis based on case report series has several limitations including publication bias, retrospective designs, lack of essential data in some cases, and others. Nevertheless, idarucizumab might augment the prothrombotic state to some extent by quick reversal of the anticoagulant effects of dabigatran and might deteriorate neurological deficits, especially when the pre-treatment aPTT is not high enough. The findings raise the question whether the reversal of dabigatran effect prior to IVT is necessary for patients with such a subnormal aPTT.
Revised clinical guides on stroke thrombolysis for anticoagulated patients in Japan
As compared to numerous case reports from Europe on stroke IVT after administration of idarucizumab, similar reports have not been published from Japan up to February 2018. A postmarketing survey of idarucizumab in Japan during the initial 6 months of its clinical use up to May 2017 did not include any patients for emergent use prior to IVT among a total of 130 registered patients. This lack may be due to the relatively wide indication for direct IVT in Japan that patients with aPTT ≤40 seconds could undergo IVT without reversing the anticoagulant effects, or because physicians hesitated to use idarucizumab in such cases in the absence of domestic clinical guides. To overcome the potential limitation, we convened a private working group, prepared a draft for the guide on stroke IVT for anticoagulated patients, and submitted it to the official journal of the Japan Stroke Society. Finally, the society recommended us to re-edit the entire contents of the draft as the authorized clinical guides by the society by adding more experts to the writing group. Table 4 shows the headline recommendations of the guides [14].
The first major point of change from the previous version is that IVT is not recommended if the time of the last dose of DOAC is <4 hours, regardless of the level of aPTT or INR. The revision was partly based on the findings of the multicenter survey indicating that asymptomatic ICH was relatively common in patients with this time window [27].
The second major point is the complete revision of the recommendation for patients taking dabigatran as follows:
(1) IVT can be considered without pretreatment with idarucizumab if aPTT is ≤40 seconds and the time of the last dose is >4 hours. Additional mechanical thrombectomy can be considered if indicated.
(2) Direct mechanical thrombectomy can be considered without pretreatment with idarucizumab or rt-PA if eligible, when aPTT is >40 seconds or the time of the last dose is or may be ≤4 hours.
(3) Treatment with 5 g idarucizumab, followed immediately by IVT, can be considered if mechanical thrombectomy cannot be quickly performed for patients whose aPTT is >40 seconds or the time of the last dose is or may be ≤4 hours. A blood sample should be collected and sent to the laboratory immediately after initiating IVT. If aPTT is not normalized, IVT should be terminated immediately.
The strategy is also shown as a flowchart in Figure 3. We also recommend that the eligibility for idarucizumab usage can be determined based on findings of markers sensitive to the intensity of dabigatran, such as ecarin clotting time, in institutes where emergent assessment of these markers is available.
Since aPTT does not completely reflect the intensity of dabigatran, treatment with idarucizumab prior to IVT when aPTT <40 seconds would further decrease the risk of post-IVT ICH. However, we have concerns that emergent reversal of dabigatran’s effects might enhance prothrombotic states to some extent and deteriorate neurological severity, as discussed earlier. This is the major reason for us not to require idarucizumab in patients with aPTT <40 seconds.
It is instructive to compare our revised guides with an expert opinion from European researchers [53]. The European expert opinion targeted patients meeting the following criteria: (1) pretreatment with dabigatran; (2) last intake of dabigatran <24 hours (96 hours if creatinine clearance <30 mL/min); (3) acute ischemic stroke (bleeding excluded by computed tomography or magnetic resonance imaging) <4.5 hours; and (4) no additional contraindications for rt-PA. Direct mechanical thrombectomy is preferentially considered for such patients, and when they cannot receive thrombectomy, treatment with idarucizumab, followed immediately by IVT, can be considered regardless of the aPTT level. In another expert opinion from the French Study Group on Hemostasis and Thrombosis, treatment with idarucizumab prior to IVT is recommended when the concentration of dabigatran is >100 ng/mL, idarucizumab is not necessary when the concentration is <50 ng/mL, and use of idarucizumab should be discussed in terms of the individual risk/benefit ratio and the possibility of mechanical thrombectomy when the concentration of dabigatran is between 50 and 100 ng/mL [54].
In addition to recommendations for IVT after idarucizumab, we briefly refer to recommendations for IVT after other antidotes in our new guides [14]. For example, four-factor prothrombin complex concentrates refill coagulation factors II, VII, IX, and X and can prevent hematoma expansion in patients with ICH related to vitamin K antagonists [55,56]. In contrast to the role of idarucizumab for dabigatran, the effect of prothrombin complex concentrates as antidotes is non-specific, and the concentrates accordingly augment the coagulation cascade and may deteriorate neurological deficits. Thus, we do not recommend IVT after emergent reversal of prolonged INR using the concentrates at this time, although a few case reports supported the feasibility of this strategy [57,58]. Similarly, we do not recommend IVT after emergent reversal of prolonged aPTT using protamine sulfate, though a few case reports supported its feasibility [59,60].
In the present review, we do not fully introduce recommendations of mechanical thrombectomy in Japan. We recently revised Japanese guidelines for application of devices on mechanical thrombectomy (http://www.jsts.gr.jp/img/noukessen_3.pdf [in Japanese]); the English version will be published soon.
Conclusions
In this review, the details and rationale of the new clinical guides on stroke IVT for anticoagulated patients in Japan are introduced. The most unique point of the guides is the use of traditional coagulation markers such as aPTT as cut-offs for idarucizumab use before IVT. These guides are practical and to some extent economical, but they have some limitations, including lack of corroborating information from sufficient numbers of relevant cases, especially from Japan. It is expected that there will be more stroke patients treated with idarucizumab prior to IVT according to the guides. The recommendations in the present guides will be further modified based on the results of future research for the third editions of the Japanese guidelines on stroke thrombolysis that will be hopefully published in 2019.