The Migraine–Stroke Connection
Article information
Abstract
Migraine and stroke are common neurovascular disorders which share underlying physiological processes. Increased risks of ischemic stroke, hemorrhagic stroke, and subclinical ischemic lesions have been consistently found in migraineurs. Three possible associations are suggested. One is that underlying pathophysiology of migraine can lead to ischemic stroke. Second, common comorbidities between migraine and stroke can be present. Lastly, some syndromes can manifest with both migraine-like headache and cerebrovascular disease. Future studies should be targeted on bidirectional influence of migraine on different stroke mechanisms and optimal prevention of stroke in migraine patients.
Introduction
Migraine is the most common neurological disorder, affecting 10% to 15% of the adult population. There are increasing evidences for an association between migraine and vascular diseases and, in particular, between migraine and ischemic stroke, subclinical brain lesions, cardiac events, and vascular mortality. Several underlying mechanisms may harbor beneath the connection between migraine and cerebrovascular disorders.
Ischemic strokes in migraine sufferers may be categorized as cerebral infarction occurring during the course of a typical migraine with aura attack (migrainous infarction) and cerebral infarction of other cause coexisting with migraine (migraine-related stroke). In this review, we will discuss epidemiology, clinical feature and possible mechanisms of increased stroke risk in migraineurs. We will also provide evidences for managing patients with both stroke and migraine.
Migrainous infarction
Epidemiology
Migrainous infarction is defined as a stroke that occurs during an attack of migraine with aura in which aura symptoms persist for >60 minutes [1]. An ischemic brain lesion must be demonstrated by neuroimaging in the appropriate territory of symptoms. The incidence of true migrainous infarction is very low. Migrainous infarction accounts for 0.2% to 0.5% of all ischemic strokes in cross-sectional studies using large stroke registries [2-5].
Clinical features and diagnosis
Diagnosis is based on International Classification of Headache Disorders (ICHD, Table 1) [1]. The ICHD criteria require a premorbid diagnosis of migraine with aura (MA) and a prolonged typical aura attack more than 60 minutes. Visual aura is the most common symptom (82.3%), followed by sensory dysfunction and aphasia [5]. The posterior circulation is more frequently affected in patients with migrainous infarction (70.6%-82.0%) than the anterior circulation [5,6]. Presenting symptoms include visual field deficit, sensory deficits, mild hemiparesis, aphasia, and tetraparesis. Most common neuroimaging findings are small and/or multiple lesions constricted in a single vascular territory [5]. Prognosis is favorable in most cases, showing complete recovery or only minor residual symptoms [6]. Figure 1 shows a typical neuroimaging of migrainous infarction.

The diagnostic criteria of migrainous infarction in International Classification of Headache Disorders, 3rd edition beta version (ICHD-3β)
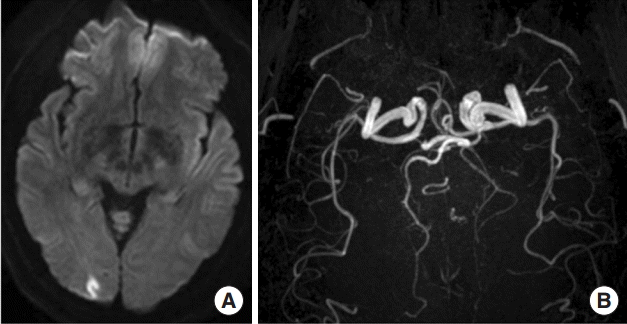
Typical manifestation and imaging findings in a patient with migrainous infarction. A 39 year-old female migraineur complained of prolonged visual aura and vertigo, which were identical with her previous migraine aura, followed by migrainous headache. She had been taking oral contraceptives for many years. Neurologic examination revealed tilting tendency to right side but it disappeared soon. (A) Diffusion MR demonstrated small cortical infarction in the right occipital lobe. (B) MR angiogram identified no steno-occlusive lesion in relevant cerebral arteries.
Potential mechanisms
Cortical spreading depression
The concept of “cortical spreading depression (CSD)” has been accepted as the main pathogenetic mechanism of migraine aura. Cerebral oligemia starts in aura phase, spreads gradually anteriorly, and is followed by hyperemia in a MA attack [7,8]. As well as MA [10], patients with migraine without aura (MO) also show posterior hypoperfusion during an attack in MR perfusion [9] and PET [10] studies. When the hemodynamic response to cortical spreading depression is inverted under pathological conditions, spreading depression can cause severe vasoconstriction instead of vasodilation [11]. However, actual incidence of migrainous infarction is very rare despite of high prevalence of migraine, therefore cerebral blood flow even during the oligemic phase of CSD seen to be above the ischemic threshold. In addition to oligemia itself, an animal CSD model shows depolarization-induced ipsilateral release of matrix metalloprotease and subsequent altered integrity of the blood brain barrier [12]. In human migraineurs, increased matrix metalloprotease is also found during migraine attacks and headache-free periods [13,14]. Taken together, CSD, which is a unique pathophysiologic mechanism of migraine aura, may precipitate an ischemic stroke.
Migraine as a risk factor for cerebral infarction (migraine-related stroke)
Epidemiology
Migraine-related stroke refers to any stroke that occurs in migraineurs [15], and its incidence rate ranges from 1.44/100,000 to 1.7/100,000 persons per year [16,17]. Migraine is an indirect or associated contributor to stroke. The Collaborative Group for the Study of Stroke in Young Women first suggested the relative risk (RR) of migraine for stroke was 2.0 [18]. Population-based studies and prospective studies consistently reported two-fold increased risk of ischemic stroke in patients with overall migraine [19-24]. Potential risk factors for ischemic stroke in migraine include younger (<45 years) age, female sex, smoking and oral contraceptive use (Table 2) [18,23]. Older (>45 years) migraineurs also show an increased risk of stroke, although the association may be either greater or less in the age over 65 [25-27]. Cigarette smoking is still an important risk enhancer after age of 45 [27]. The risk varies with the subtypes of migraine. MA has been consistently reported as a stroke risk factor, with a pooled relative risk of 2.16 (95% CI 1.53-3.03) and a population-attributable risk of 3.5% [28]. The risk increases with an increased frequency of migraine attack [29,30]. Regarding MO, no additional risk for stroke has been found from updated meta-analyses [23,24]. In men, the association with migraine and stroke is still controversial [23,31,32].
Mechanisms
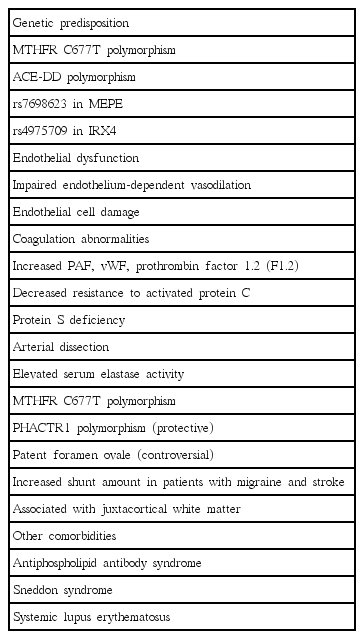
There are several hypotheses to explain the increased risk of ischemic stroke in migraine sufferers. First, migraine itself can predispose to ischemic stroke. Second, common comorbidities between migraine and stroke can be present. Third, some genetic disorders can manifest with both migraine and cerebrovascular disease. Lastly, migraine-specific medication, especially vasoconstrictors, can be associated with increased risk of stroke. We will further discuss specific pathogenic mechanisms between migraine and stroke (Table 3).
Genetic associations
The genetic association between ischemic stroke and MA has been suggested. Polymorphism in the MTHFR (methylenetetrahydrofolate reductase) gene is the candidate gene with mediates increased risk of ischemic stroke in MA [33,34]. MTHFR gene encodes for a key enzyme for the metabolism of folate and homocysteine and is associated with susceptibility to MA [33,35,36]. Angiotensin-converting enzyme gene deletion polymorphism (ACE-DD) is another possible connection. ACE-DD is related with von Willebrand factor (vWF), venous thrombophilia, hypercoagulability, increased vascular smooth muscle tone, and lacunar infarction [37]. The ACE-DD polymorphism is also found in migraine and associated with increased frequency of attacks [38,39]. Genome-wide association studies revealed genetic overlap between migraine, ischemic stroke, and coronary artery diseases [40,41]. The genetic overlap was stronger with large-artery and cardioembolic subtypes and MO subtype [41]. As of 2015, 38 susceptibility loci have been identified, which linked to ion homeostasis, nitric oxide signaling and oxidative stress, previous vascular disease associations, vascular tissues and brain tissues [40].
Endothelial dysfunction
Endothelial dysfunction might play a role connecting migraine and stroke. Endothelial dysfunction is characterized by reduction in vasodilator activities, increase in endothelial-derived vasoconstrictors, and consequent impairment of the vascular reactivity of the vasculature. It can lead to a procoagulatory, proinflammatory and proliferative state, which predisposes to atherosclerosis. Biomarkers of endothelial dysfunction, i.e., elevated vWF antigen, vWF activity, high-sensitivity C-reactive protein and reduced nitrate/nitrite levels, were found in migraineurs [42,43]. Circulating endothelial progenitor cell (EPC) studies showed that the number and function of EPCs were decreased and negatively correlated with longer disease duration in migraine [44,45]. Another study revealed that total number of EPC was not reduced but the number of activated EPCs was higher in migraineurs [46]. Circulating endothelial microparticles are also elevated in women with MA, suggesting that endothelial activation might be involved in migraine pathophysiology [47]. It is of interest whether endothelial dysfunction is present in systemic or cerebral vasculature. Systemic endothelial dysfunction has been widely tested in migraine with forearm blood flow by venous plethysmography, flow-mediated dilatation, and pulse-wave velocity analyses, yielding conflicting results [48-53]. In contrast, cerebral vascular reactivity has been consistently reported to be decreased in migraine, especially in the posterior circulation [51,52]. It seems that there is a lack of association between systemic and cerebral endothelial dysfunction in migraine [54]. In the future, the clinical significance of cerebral endothelial dysfunction and therapeutic implications should be tested for reducing stroke risk of migraineurs.
Coagulation abnormalities
Platelet aggregation and increased levels of platelet-activating factor (PAF) and vWF are observed during migraine attacks [55-57]. PAF is released from cerebral endothelial cells, platelets and mast cells in response to hypoxia and calcitonin gene-related peptide (CGRP), and in turn, prompts the release of vWF. vWF indirectly activates the platelet IIb/IIIa receptor and leading to hemostasis. Other reported that coagulation abnormalities–elevated prothrombin factor 1.2, decreased resistance to activated protein C, and protein S deficiency–are present in patients with MA [58,59]. It is not clear whether plasma hypercoagulability is either the result or the cause of CSD.
Arterial dissection
Increased incidence of spontaneous cervical carotid or vertebral artery dissection was found in patients with migraine [60-62]. In a meta-analysis, migraine is associated with a two-fold increased risk of cervical artery dissection (pooled OR=2.06, 95% CI 1.33-3.19) [63]. Among the migraine subtypes, MO has the highest risk for dissection in most studies [60,61]. Elevations of serum elastase activity and shared genetic alterations such as MTHFR polymorphism are possible mechanisms [64,65]. A recent genomewide association study of cervical arterial dissection identified a significant association of the PHACTR1 locus, which is also associated with migraine [66], suggesting the possibility of shared genetic components. Intracranial arterial dissection has not been investigated in association with migraine.
Patent foramen ovale
Patent foramen ovale (PFO) is a common congenital cardiac defect and may serve as right-to-left shunt for paradoxical embolism. PFO is twice as frequent in patients with MA, and MA is twice as frequent in patients with PFO, than in controls [67,68]. Several studies were performed to reveal the actual relationship of PFO and MA. Although some retrospective studies reported that PFO closure has beneficial effects on migraine [69,70], a recent population-based observational study and a large case-control study demonstrated lack of association [71,72]. Furthermore, a double-blind, randomized clinical trial of PFO closure for migraine showed no beneficial effect on the cessation of migraine [73]. In conclusion, it is less likely that PFO plays a role for developing migraine headache.
Nevertheless, the role of PFO in developing ischemic stroke in migraineurs is not determined yet. Alzola et al reported that patients with both migraine and stroke had larger shunts than did patients with migraine without stroke, patients with no migraine with stroke, and controls [74]. Regarding the white matter hyperintensities (WMH), overall WMH did not differ by the presence of PFO [75]. However, juxtacortical WMH are more frequently found in patients with migraine and associated with right-to-left shunt [76]. These findings suggest that coincidental PFO might enhance the risk of ischemic stroke in migraineurs.
Other comorbidities
Antiphospholipid (aPL) antibody syndrome [77], Sneddon syndrome [78], and systemic lupus erythematosus [79] are associated with both MA and stroke. Antiphospholipid antibody is a risk marker for stroke in young women. People with primary and secondary aPL antibody syndrome present with headache and transient focal neurologic events. Investigation of PFO, MVP, coagulopathy, and aPL antibody might be helpful in patients with migraine-related stroke.
Migraine and subclinical brain lesions
WMH are more frequently found on neuroimaging in migraineurs than in non-migraine population. Earlier meta-analysis provided high odds ratio with 3.90 (95% CI 2.26-6.72) for having WMH in migraineurs [80]. Recent meta-analysis estimated lower odds (OR 1.68, 95% CI 1.07-2.65) and showed the association only in MA, not in MO [81]. Subclinical brain infarcts were found more in migraineurs than in controls, particularly in the posterior circulation [82-84]. Increased risk of having WMH or subclinical infarcts is associated with increased headache frequency [84,85]. Longitudinal MRI follow-up studies showed that WMH increase over time in migraineurs [86,87]. However, large population-based prospective studies failed to document temporal associations between the number of migraine headache attacks and brain lesion progression [88,89]. Clinical significance of WMH seen in migraine patients is still unclear. Cognitive dysfunction seems not to be associated with WMH in migraineurs [90,91].
Migraine and hemorrhagic stroke
In addition to higher risk of ischemic stroke, migraineurs also have a risk for developing hemorrhagic stroke. Women’s Health Study demonstrated increased risk for hemorrhagic stroke in women with active migraine with aura (adjusted HR 2.25, 95% CI 1.11-4.54) [92]. A meta-analysis revealed that the risk of hemorrhagic strokes was greater in females with any types of migraine and in female migraineurs aged less than 45 years [93]. However, in a recent population-based study, the risk of hemorrhagic stroke increased irrespective of sex or age group (<45 years and ≥45 years) [94]. Types of hemorrhagic strokes included subarachnoid hemorrhage and intracerebral hemorrhage [93,94]. Most of studies did not provide information about the presence of aneurysm. Mechanisms underlying the association between migraine and hemorrhagic stroke are unclear. Although the association is positive in public health aspect, the absolute incidence of hemorrhagic stroke is very low in general practice [95].
Hereditary conditions that cause both stroke and migraine
Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL)
Migraine and ischemic stroke are common features of CADASIL, which is a disorder of small cerebral arteries [96]. Transient ischemic attacks and ischemic strokes are the most frequent manifestations in CADASIL, occurring in 60%-85% of patients [97-99]. Migraine with aura is reported in 20%-50% of patients with CADASIL, which is five times greater than in the general population. In a Korean cohort of CADASIL, headache is the most frequent symptom with a prevalence of 45.3%, followed by cerebral infarction [100]. Headache is usually the first symptom, with an average age at onset of 30 years [101]. The nature of headache is generally similar to those of classic migraine [100]. However, older age of onset and higher frequency of atypical aura can be found in patients with CADASIL [101].
Notch-3 mutation is the gene involved in CADASIL, encoding a transmembrane receptor primarily expressed in systemic arterial smooth-muscle cells [102]. Genetic testing is the gold standard for the diagnosis of CADASIL, showing very high sensitivity and specificity, reaching 100% [103]. Skin biopsy can be helpful. Presence of granular osmiophilic material on electron-microscopic study of skin biopsy samples indicates arteriopathy of CADASIL, but the sensitivity is variable [104]. MRI can present abnormalities prior to clinical onset. Characteristic imaging findings are T2 hyperintense lesions in the temporal pole, external capsule and corpus callosum [105].
Treatment of migraine with aura in CADASIL patients is generally not different with that of non-CADASIL migraineurs. Prophylactic treatment is rarely required as the frequency of attacks is low in most cases. If required, the usual prophylactic drugs such as antiepileptic drugs or beta blockers can be used. For acute treatment, vasoconstrictors such triptans or ergot derivatives should be avoided.
Mitochondrial Encephalopathy, Lactic Acidosis and Stroke-like episodes (MELAS)
MELAS is a genetically heterogeneous mitochondrial disorder characterized by features of central nervous system involvement (seizures, hemiparesis, hemianopia, cortical blindness, sensorineural deafness, and/or episodic vomiting). Headache is a common manifestation, which is either a recurrent migraine-like attack or a presenting symptom of stroke-like episodes [106].
Migraine abortive drugs and the risk of stroke
Acute abortive medications for migraine attacks (e.g. ergotamine and triptans) have vasocontrictive actions. Thus, some clinicians too much concern of cerebral vasospasm and consequent stroke when prescribing these agents. However, stroke rarely occurs in migraine patients who do not abuse ergots or triptans in the absence of contraindication.
Ergot derivatives
Ergotamine and dihydroergotamine are nonselective 5-HT1 agonists which have affinities for dopamine and noradrenaline receptors [110]. Ergotamine in excessive dosage is often associated with cardiovascular events involving the coronary arteries and peripheral vascular system or also may cause cerebral vasospasm [111,112]. Drug interaction with cytochrome P450 3A4 enzyme inhibitors (Macrolide antibiotics–Erythromycin and Clarithromycin; antifungal agents–Ketoconazole, Itraconazole, etc; protease inhibitors–Ritonavir etc; and antidepressants–Nefazodone, Fluoxetin and Fluvoxamine) may potentially increase the risk of cerebral ischemia [113]. Although ergot is contraindicated in patients with history of stroke [110], it is not evident that ergot therapy can cause stroke at standard doses. So far, use of ergot alkaloids in migraine patients has not been statistically verified as a stroke risk factor [111,114].
Triptan (Selective 5-HT1B/1D agonist agent)
Triptans may present with stroke-like events but there is no convincing evidence. In several studies, triptan treatment is not associated with increased risk of stroke, even in the setting of overuse [111,115]. Sumatriptan, the prototype of triptan, has once been reported to cause cerebral infarction in a patient with sinus thrombosis [116]. A case of spinal cord infarction was reported during zolmitriptan use [117]. In two population-based studies, however, there was no evidence that triptans lead an increased risk of vascular events [114,115].
Clinical pearls for managing migraine in patients with ischemic stroke
Treatment of migraine in patients with stroke
Some new antiplatelet agents and vasodilators can cause migraine-like headaches. Cilostazol, an antiplatelet drug that inhibits phosphodiesterase 3, has also vasodilatory activity. In CSPS-2 trial, 313 of 1,337 (23%) patients taking cilostazol complained for headache [118]. In a study using healthy volunteers without primary headache, 11 of 12 volunteers reported cilostazol-induced headache, that were characterized by mild to moderate intensities, bilateral locations and pulsating nature [119]. Cilostazol can induce migraine-like attacks in migraineurs, which respond to migraine treatment [120]. To reduce the development of headache, cilostazol can be started with lower dose, and then escalate the dose with interval of 1-2 weeks. Dipyridamole, a phosphodiesterase 5 inhibitor, has been reported to induce migraine in patients with migraine without aura [121].
There are a few anecdotal reports of efficacy of antiplatelet agents or warfarin for reducing migraine attacks [122,123], particularly in patients with PFO [124]. However, a recent small randomized controlled trial failed to prove efficacy of clopidogrel for migraine prophylaxis [125].
Antihypertensive agents are frequently prescribed among patients at risk of ischemic stroke. Beta-adrenergic blocker and calcium channel blocker are well established treatment for preventing migraine [126,127]. Some angiotensin receptor blockers and angiotensin-converting-enzyme inhibitors has been reported as effective for prevention of episodic migraine. Candesartan has shown significantly better effect than placebo in reducing frequency, severity, and disability of migraine [128]. Another randomized, double blinded, placebo-controlled study has shown that olmesartan reduced frequency and severity of migraine attacks [129]. Lisinopril has favorable effect on the reduction of frequency, duration and severity in migraine attacks [130].
Statin has been reported to be efficacious for migraine prophylaxis in a case report using atorvastatin 20 mg [131] and a small open-label prospective study using simvastatin 20 mg [132]. A recent randomized controlled trial showed effectiveness of simvastatin 20 mg combined with vitamin D for preventive treatment of migraine [133]. The positive result is supported by preclinical evidences that statin reduces expression of CGRP and substance P and attenuates NF-κB activation in trigeminal nucleus caudalis [134,135].
Prevention of stroke in migraineurs
Currently, there are no recommendations of pharmacotherapy for the primary prevention of ischemic stroke in migraineurs. Risk factor modification is a preferred method of primary prevention. Smoking cessation and abstinence from oral contraceptives are important. Antithrombotics are not recommended to reduce a risk of stroke in migraineurs. Antihypertensives and statins should be given with a proper indication. If needed, the selection of medications which have efficacy for migraine prophylaxis may be beneficial to reduce both migraine attacks and vascular risks in migraineurs.
Conclusion
In summary, an increased association with various types of stroke is present in migraineurs. Stronger association with stroke is present in MA with a possible causal impact via CSD. MO might not have a direct causal relationship to lead stroke, but share genetics, risk factors and comorbidities in common with stroke. Genetic syndromes can cause both migraine-like headache and stroke. Medications are another important point of patient care. The association and interaction between migraine drugs and stroke risks, and vice versa, should be considered for optimal patient management.
Notes
The authors have no financial conflicts of interest.