Folic Acid in Stroke Prevention in Countries without Mandatory Folic Acid Food Fortification: A Meta-Analysis of Randomized Controlled Trials
Article information
Abstract
Background and Purpose
Additional folic acid (FA) treatment appears to have a neutral effect on reducing vascular risk in countries that mandate FA fortification of food (e.g., USA and Canada). However, it is uncertain whether FA therapy reduces stroke risk in countries without FA food fortification. The purpose of this study was to comprehensively evaluate the efficacy of FA therapy on stroke prevention in countries without FA food fortification.
Methods
PubMed, EMBASE, and clinicaltrials.gov from January 1966 to August 2016 were searched to identify relevant studies. Relative risk (RR) with 95% confidence interval (CI) was used as a measure of the association between FA supplementation and risk of stroke, after pooling data across trials in a random-effects model.
Results
The search identified 13 randomized controlled trials (RCTs) involving treatment with FA that had enrolled 65,812 participants, all of which stroke was reported as an outcome measure. After all 13 RCTs were pooled, FA therapy versus control was associated with a lower risk of any future stroke (RR, 0.85; 95% CI, 0.77 to 0.95). FA alone or combination of FA and minimal cyanocobalamin (≤0.05 mg/day) was associated with a lower risk of future stroke (RR, 0.75; 95% CI, 0.66 to 0.86) whereas combination of FA and cyanocobalamin (≥0.4 mg/day) was not associated with a lower risk of future stroke (RR, 0.95; 95% CI, 0.86 to 1.05).
Conclusions
FA supplement reduced stroke in countries without mandatory FA food fortification. The benefit was found mostly in patients receiving FA alone or combination of FA and minimal cyanocobalamin.
Introduction
Homocysteine, a sulfur-containing amino acid, was thought to be an independent vascular risk factor [1]. Hyperhomocysteinemia was related with endothelium dysfunction and procoagulation state through mechanisms of altering vascular morphology, stimulating inflammation, activating the endothelium and the blood clotting cascade, and inhibiting fibrinolysis [2]. A 25% lower usual total homocysteine level was associated with a 19% lower stroke risk [1]. Folic acid, as a cofactor of enzymes involved in homocysteine metabolism, could lower total homocysteine by 20% to 25% [3], and it was supposed to decrease the risk of vascular events. Many randomized controlled trials (RCTs) had been conducted to evaluate the efficacy of folic acid supplementation for the prevention of cardiovascular/cerebrovascular diseases, but their results were diverging.
To our knowledge, meta-analyses regarding this issue mostly found no benefit of folic acid for coronary artery disease prevention [4,5]. However, there might be slight benefits in terms of stroke prevention [5-8]. A meta-analysis found folic acid supplementation reduced the stroke risk by 11% in the 10 trials with no or partial folic acid fortification but had no benefit on stroke risk in the other five trials with folic acid mandatory fortification [7]. Indeed, a population-based cohort study had found stroke mortality decreased in United States and Canada after mandatory folic acid fortification [9]. Therefore, additional folic acid supplementation in these countries may have limited benefit to further reduce stroke risk. On the other hand, in the countries without mandatory folic acid fortification, weather folic acid supplementation could prevent stroke would be an important issue for public health.
Some RCTs carried out in the countries without folic acid fortification have been published in the interval since the most recent meta-analysis and offered more evidence on this issue [10,11]. Therefore, we undertook an update meta-analysis to evaluate the efficacy of folic acid therapy on stroke prevention in countries without mandatory folic acid food fortification.
Methods
This study was performed in accordance with the recommendations of the preferred reporting items for systematic reviews and meta-analysis: the PRISMA statement [12].
Search strategy
We searched PubMed, EMBASE, and the clinical trial registry maintained at clinicaltrials.gov from January 1966 to September 2016 using the search strategy “homocysteine” or “folate” or “folic acid” or “vitamin B12” or “cobalamin” or “vitamin B6” or “pyridoxine” or “multivitamin” and “cardiovascular disease” or “myocardial infarct” or “myocardial ischemia” or “coronary heart disease” or “angina” or “heart attack” or “stroke” or “cerebrovascular disease” or “cerebrovascular attack” or “brain attack” or “brain infarct” or “brain hemorrhage” or “intracranial hemorrhage." We restricted our search to human beings and clinical trials. There were no language restrictions. We also reviewed the introduction and discussion sections of retrieved trials and of prior meta-analyses to identify additional trials.
Included criteria for studies were as follows: (1) the study designed as an RCT; (2) the active treatment comprised folic acid supplementation (with or without additional vitamin B supplementation); (3) total participants and number of stroke events were reported as an outcome endpoint; (4) duration of active treatment at least 6 months; and (5) most participants (>50%) in a trial resided in countries without mandatory folic acid food fortification. Participants of any age or sex were included.
Since mandatory folic acid supplement has been applied to United States (since 1998), Canada (since 1998), Costa Rica (since 1998), Chile (since 2000), and South Africa (since 2003), trials with >50% of participants resided in these countries were excluded.
All data from eligible trials were abstracted in duplicate by two investigators independently (C.Y.H. and Y.L.W.) with a standard protocol. Discrepancies were resolved by discussion with a third investigator (M.L.) and by referencing the original report.
Study quality assessment
Since all the included studies were RTCs, the risk of bias (e.g., selection bias, performance bias, detection bias, attrition bias, and reporting bias) of the included trials was assessed by Cochrane risk-of bias algorithm (www.cohchrane.org/training/cochrane-handbook) [13].
Primary and secondary endpoints
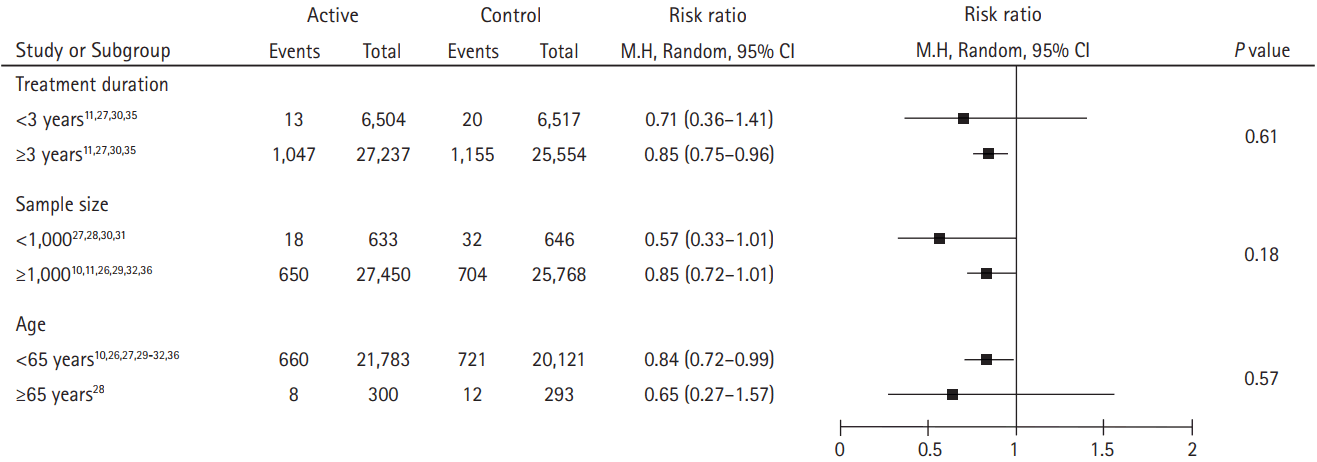
The primary endpoint was stroke (ischemic or hemorrhagic). The secondary endpoints were ischemic stroke and hemorrhagic stroke, respectively. Subgroup analyses were conducted according to different study characteristics: treatment duration (<3 years vs. ≥3 years), sample size (<1,000 vs. ≥1,000), and mean age at entry (<65 years vs. ≥65 years).
Statistical analysis
All analyses were based on the intention-to-treat principle. Relative risk (RR) with 95% confidence interval (CI) was used as a measure of the association between folic acid supplementation and risk of stroke. We report absolute risks in terms of the difference in the number of events per 1,000 patients and the respective 95% CI. We computed a random-effects estimate based on the Mantel-Haenszel method when two or more studies provided sufficient data for a given outcome and compared the results with those obtained from a fixed-effects model. Statistical heterogeneity was assessed by the chi-square and the I2 statistics. Heterogeneity was considered if either the chi-square test was significant with the P=0.10 level, or the I2 statistic was greater than 50%. Publication bias was estimated visually by funnel plots displaying standard error as the measure of sample size and RR as the measure of treatment effect [14]. We also performed a sensitivity analysis to further explore the robustness of our results. To identify any study that might have exerted a disproportionate influence on the summary treatment effect, we removed each individual trial from the meta-analysis one at a time. Subgroup analyses were conducted based on the baseline characteristics. This meta-analysis was analyzed by Cochrane Collaboration’s Review Manager Software Package (RevMan version 5.3, The Cochrane Collaboration, London, UK).
To evaluate whether the present meta-analysis had sufficient sample size to reach firm conclusions about the effect of interventions, trial sequential analysis was performed for the primary endopint [15]. Trial sequential analysis performs accumulative meta-analysis, which creates Z curve of the summarized observed effect and the monitoring boundaries for benefit, harm, and futility, and it estimates the required information size. These boundaries and analyses are adjusted to account for the amount of available evidence and to control for repeated analyses, while maintaining type I error at 5% and the power at 80%. The required information size was calculated based on event rates observed in control group and folic acid group. If the Z curve of the cumulative meta-analysis crosses one of the boundaries, no further studies are required, and there is sufficient evidence to support the conclusions.
Results
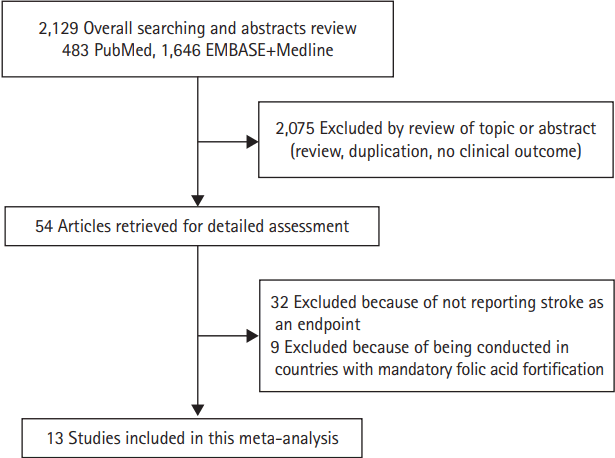
The literature review identified 54 articles for detailed assessment, among which 32 were excluded for not reporting stroke as an endpoint, nine were excluded for being conducted in countries with mandatory folic acid fortification [16-24], and our final analysis included 13 RCTs, conducted in countries without mandatory folic acid fortification, involving treatment with folic acid (Figure 1) [10,11,25-35]. Included [10,11,25-35] versus excluded trials [16-24] based on whether they were conducted mostly in countries without mandatory folic acid fortification were presented in Table 1. Study design characteristics and baseline characteristics of included trials were presented in Table 2 [10,11,25-35]. Overall, 65,812 participants were enrolled with 33,741 (51%) participants randomly assigned to the folic acid therapy group and 32,071 (49%) to the control group. Among 13 trials, 12 trials included individuals with preexisting conditions: stroke (one trial) [33], coronary heart disease (five trials) [26-28,31,35], cardiovascular disease (one trial) [32], end-stage renal disease or advanced chronic kidney disease (three trials) [29,30,34], hypertension (one trial) [10], and esophageal dysplasia (one trial) [25] while one trial included individuals living in high altitude [11]. Folic acid alone or combination of folic acid and minimal cyanocobalamin (≤0.05 mg/day) was used in an active treatment group in eight trials [10,25-27,29,30,32,34] whereas combination of folic acid and cyanocobalamin (≥0.4 mg/day) was used in an active treatment group in five trials [11,28,31,33,35]. Three trials excluded participants with chronic kidney disease [26,27,32] while other trials included some participants with chronic kidney disease. Cerebrovascular events analyzed were combined nonfatal and fatal strokes (ischemic and hemorrhagic) in 10 trials; for one trial each, data were available only on fatal stroke [25]; non-fatal and fatal ischemic stroke [11]; and the composite of nonfatal and fatal stroke plus transient ischemic attack [27]. Neuroimaging was explicitly mentioned as part of the stroke event ascertainment process in seven trials [10,11,28,29,31,34].

Included versus excluded trials based on whether they were conducted mostly in countries without mandatory folic acid fortification
Risk-of-bias assessment of included trials was reported in Supplementary Table 1. The results from Liem et al. [26], Liem et al. [27], and Righetti et al. [29] had high risks of performance bias and detection bias because these were an open, non-blinded studies.
After all 13 trials were pooled, folic acid therapy versus control was associated with a lower risk of any future stroke (RR, 0.85; 95% CI, 0.77 to 0.95; P=0.004; 3 to 8 fewer events/1,000 patients). Folic acid alone or combination of folic acid and minimal cyanocobalamin (≤0.05 mg/day) was associated with a lower risk of future stroke (RR, 0.75; 95% CI, 0.66 to 0.86; P<0.0001) whereas combination of folic acid and cyanocobalamin (≥0.4 mg/day) was not associated with a lower risk of future stroke (RR, 0.95; 95% CI, 0.86 to 1.05; P=0.30) (Figure 2). There was slightly asymmetrical appearance on the funnel plot suggesting a small degree of publication bias, with a slight under-representation of studies showing neutral effects (Supplementary Figure 1). Sensitivity analyses excluding individual trials yielded pooled results that were not significantly different from the overall pooled estimates (Table 3). Trial sequential analysis was conducted and showed the number of patients evaluated (n=65,812) almost reached the required information sizes (n=66,784) and the summarized observed effect crossed benefit boundary (Figure 3).
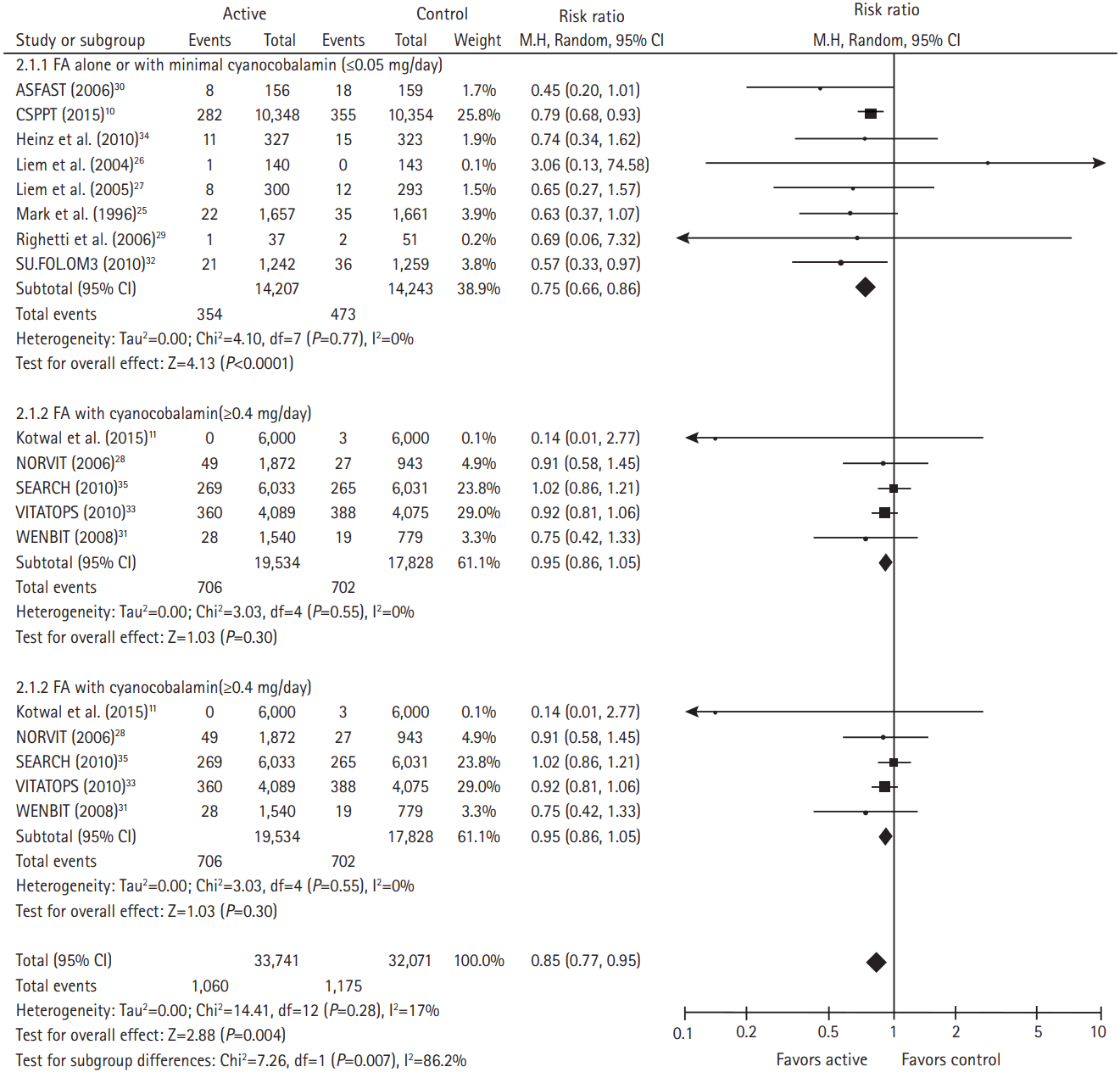
Risk ratio with 95% CI estimates for stroke (active treatment vs. control), by trial and pooled. M-H, Mantel-Haenszel methods; CI, confidence interval; FA, folic acid; ASFAST, Atherosclerosis and Folic Acid Supplementation Trial; CSPPT, China Stroke Primary Prevention Trial; SU.FOL.OM3, SUpplementation with FOlate, vitamin B6 and B12 and/or OMega-3 fatty acids; NORVIT, Norwegian Vitamin; SEARCH, Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine; VITATOPS, VITAmins TO Prevent Stroke; WENBIT, Western Norway B-Vitamin Intervention Trial.
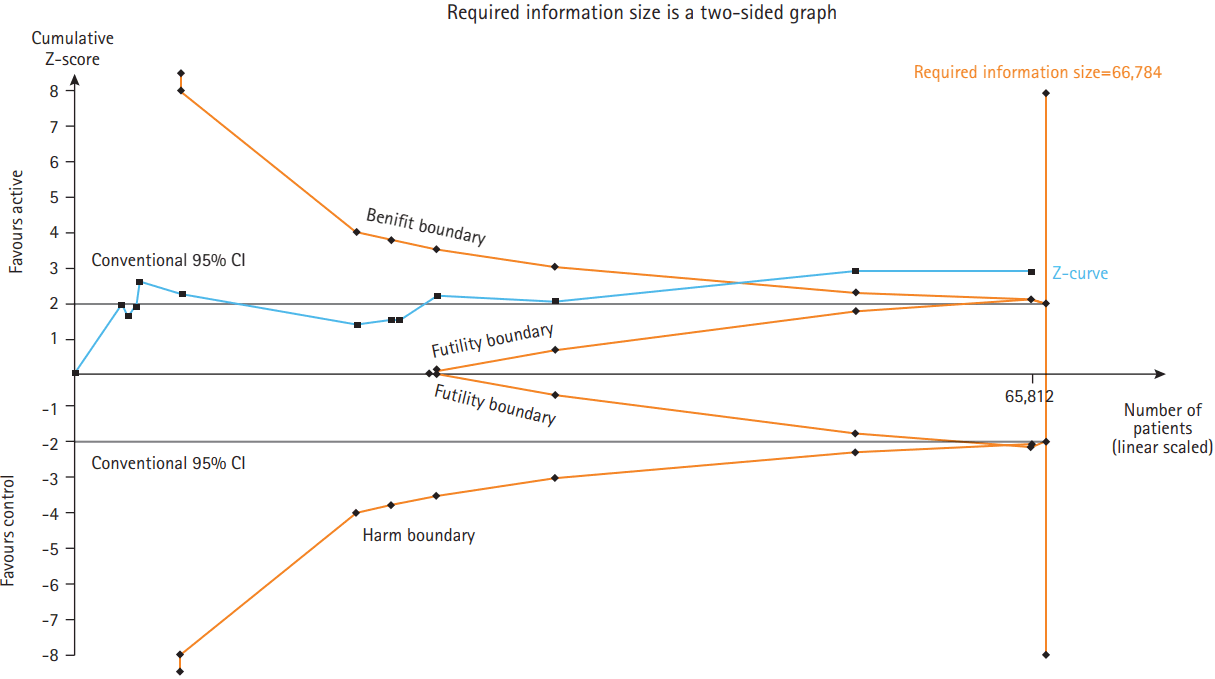
Trial sequential analysis of 13 trials comparing folic acid and control for stroke in countries without mandatory folic acid fortification. A cumulative Z-curve almost reached the required information size boundary and has crossed benefit boundary. CI, confidence interval.
Two trials reported data on ischemic stroke and one trial reported data on hemorrhagic stroke. There was no evidence of an effect of folic acid on ischemic (RR, 0.65; 95% CI, 0.25 to 1.71; P=0.38) and hemorrhagic stroke (RR, 0.94; 95% CI, 0.66 to 1.34; P=0.72), respectively. The overall qualities of evidence were high in stroke endpoint and low in ischemic stroke (Supplementary Table 2).
In subgroup analyses, folic acid therapy was associated with a reduction or decreasing trend in the risk of subsequent strokes when we stratified the estimates by treatment duration, sample size, and mean age at entry. There was also no obvious heterogeneity among the different study characteristics (Figure 4).
Discussion
In this meta-analysis of 13 RCTs of generally good quality, among over 65,000 people living in countries without mandatory folic acid fortification in food, we found that addition of folic acid was associated with a 15% RR reduction in future stroke risk. Folic acid alone or combination of folic acid and minimal cyanocobalamin (≤0.05 mg/day) was associated with a 25% lower risks of future stroke whereas combination of folic acid and cyanocobalamin (≥0.4 mg/day) was not associated with lower risks of future stroke. While five fewer stroke events/1,000 patients may seem modest, additional folic acid supplementation may be associated with huge benefits with inexpensive cost, since most countries have not adopted mandatory folic acid fortification. This updated meta-analysis provided robust evidence of beneficial effects of folic acid supplementation for stroke prevention in countries that do not have mandatory folic acid fortification in food.
The finding of no benefits in trials with patients receiving folic acid plus cyanocobalamin (≥0.4 mg/day) deserved further exploration [11,30,31,33,35]. All these trials included some participants with chronic kidney disease [11,28,31,33,35]. In the DIVINe trial [22], in patients with diabetic nephropathy, B vitamins including cyanocobalamin 1,000 mcg daily were harmful, accelerating the decline of renal function, and doubling cardiovascular events. All the events occurred among participants with estimated glomerular filtration rate <50 mL/min/1.73 m2 [36]. In the Vitamin Intervention for Stroke Prevention (VISP) subgroup analysis [37] that excluded participants with estimated glomerular filtration rate <48 mL/min/1.73 m2 (the lowest 10%), there was a significant benefit of B vitamins including cyanocobalamin. It now appears that the reason the early studies that did not show a reduction of stroke (e.g., VISP, Norwegian Vitamin [NORVIT]) was because high cyanide levels in patients with renal failure resulted in toxicity of cyanocobalamin [38,39]. On the other hand, the benefit of folic acid monotherapy was found in the huge Chinese trial (China Stroke Primary Prevention Trial [CSPPT)], even among participants with impaired renal function [40]. Furthermore, in countries with folate fortification the main nutritional cause of high total homocysteine is metabolic B12 deficiency, which is very common (30% of stroke patients over age 70) and often missed [41]. The reason it is missed is that a normal serum total B12 does not confirm adequate levels of active cobalamin; to do so requires measuring holotranscobalamin, methylmalonic acid or total homocysteine [42]. It has been suggested that metabolic B12 deficiency should be investigated and treated in all stroke patients. Methylcobalamin or hydroxocobalamin should be used, particularly in patients with renal impairment [38].
The different effect of folic acid on myocardial infarction and stroke is worthy of comment. Myocardial infarction is mostly due to plaque rupture in the coronary artery and its mechanism is large vessel atherosclerosis. The mechanisms of stroke are more heterogeneous, including cardioembolism, large vessel atherosclerosis and small vessel disease. Within the stroke subtypes, small vessel disease was found to have strongest association with total homocysteine in many populations [43-46]. Total homocysteine was also related to silent brain infarct and white matter lesions on magnetic resonance imaging of brain [47-50]. Beyond cerebral small vessel disease, total homocysteine has also been related to small vessel disease in other vascular beds, including in chronic kidney disease and diabetic retinopathy [51,52]. In fact, some studies have indicated that total homocysteine-lowering therapy with vitamin B may slow the progression of white matter lesions of brain [53,54]. Given all of the aforementioned data, we think that total homocysteine-lowering therapy might provide more benefit on small vessel disease than large vessel atherosclerosis, and would provide a larger protective effect on overall stroke versus myocardial infarction.
Folic acid food fortification program was introduced in United States and Canada since 1998 for prevention of neural tube defect in newborn [55]. The US program added 140 μg of folic acid per 100 g of enriched cereal grain product and has been estimated to provide 100 to 200 μg of folic acid per day to women of childbearing age [55]. Study found the mean folate concentrations increased from 4.6 to 10.0 ng/mL and the mean total homocysteine concentration decreased from 10.1 to 9.4 μmol/L after folic acid fortification [56]. Moreover, the prevalence of low folate concentrations (<3 ng/mL) decreased from 22% to 1.7% and the prevalence of high total homocysteine concentrations (>13 μmol/L) decreased from 18.7% to 9.8% after folic acid fortification [56]. Epidemiology study found the stroke mortality improved in United States and Canada after folic acid fortification [9]. A dose-finding trial found folic acid dose as low as 0.2 mg/day, if given for more than 6 months, effectively lowered total homocysteine concentration by 8% to 20% as higher dose (0.4 and 0.8 mg/day) [57]. The study also found folic acid 0.8 mg/day achieved maximal total homocysteine-lowering response by 6 weeks but then the total homocysteine level remained stationary by 6 months [57]. Therefore additional folic acid supplementation in the countries with folic acid food fortification may not reduce stroke risk further. A case-control study found in the era of folic acid fortification, vitamin B6 had stronger association with stroke than total homocysteine [58]. Therefore, in the countries with folic acid fortification, other risk factors other than total homocysteine may be more important for the prevention of stroke.
Although this meta-analysis included only RCTs, there were some limitations. First, meta-analysis is retrospective research that can be constrained by the comprehensiveness of searches, methodological rigor of the included studies, and publication bias. We tried to maximize study identification and minimize bias by developing the study protocol a priori, performing a thorough search of several databases, and using explicit criteria for study selection, data collection, and data analysis. Second, inevitable bias lies in the different characteristics of study participants, treatment duration and intensity, type of cerebrovascular events identified, baseline folate and total homocysteine concentration, percentage of concomitant antiplatelet or statin use and other study design variables. Third, since only two trials reported an endpoint of ischemic stroke and one trial reported an endpoint of hemorrhagic stroke, insufficient evidence can be obtained on these endpoints.
Conclusions
In conclusion, our meta-analysis demonstrated a significant benefit of folic acid supplement in preventing stroke in countries without mandatory folic acid food fortification. The benefit was found mostly in patients receiving folic acid alone or combination of folic acid and minimal cyanocobalamin (≤0.05 mg/day) but not found in trials with combination of folic acid and cyanocobalamin (≥0.4 mg/day). Since the number of patients evaluated stroke of current RCTs almost reached the required information sizes and the summarized observed effect has crossed benefit boundary, no more relevant RCTs are needed to be conducted in countries without mandatory folic acid fortification. Because folic acid supplementation is an inexpensive, safe, and widely applicable intervention, a nutritional supplementation of folic acid by food or medication should be promoted in countries where food was not fortified with folic acid.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2017.01522.
Risk-of-bias assessment of included trials
Summary of quality assessments and findings for primary and secondary outcomes
Funnel plot. SE, standard error; RR, relative risk.
Notes
Disclosure
The authors have no financial conflicts of interest.