Basilar Artery Plaque and Pontine Infarction Location and Vascular Geometry
Article information
Abstract
Background and Purpose
Subclinical atherosclerotic plaques are common in patients with pontine infarctions (PIs) but without basilar artery (BA) stenosis. We hypothesized that BA plaque locations may differ by PI type and vertical location as well as vertebrobasilar artery geometry.
Methods
Ninety-six patients with PI but without BA stenosis on magnetic resonance imaging (MRI) and magnetic resonance angiography were enrolled. PIs were classified by type (paramedian, deep, or lateral) and vertical location (rostral, middle, or caudal). Patients underwent high-resolution MRI to evaluate BA plaque location (anterior, posterior, or lateral). The mid-BA angle on anteroposterior view and angle between the BA and dominant vertebral artery (BA-VA angle) on lateral view were measured.
Results
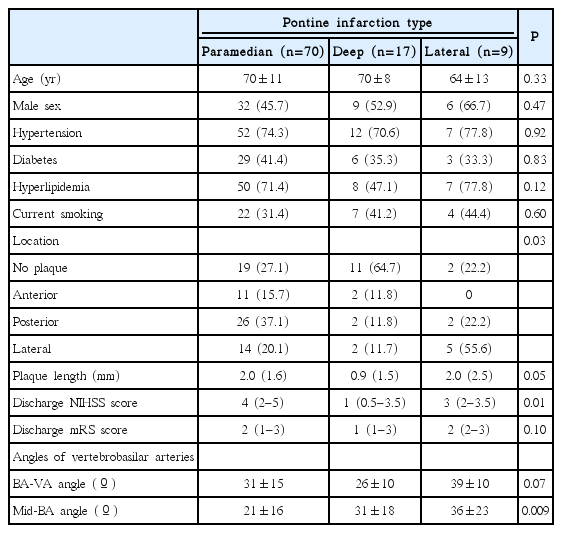
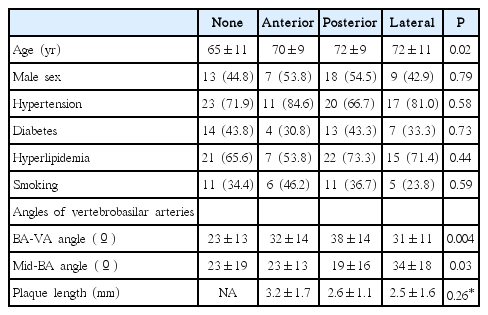
The PIs were paramedian (72.9%), deep (17.7%), and lateral (9.4%) type with a rostral (32.3%), middle (42.7%), and caudal (25.0%) vertical location. The BA plaque locations differed by PI type (P=0.03) and vertical location (P<0.001); BA plaques were most frequent at the posterior wall in paramedian (37.1%) and caudal (58.3%) PIs and at the lateral wall in lateral (55.5%) and middle (34.1%) PIs. The BA-VA and mid-BA angles differed by BA plaque and PI vertical location; the greatest BA-VA angle was observed in patients with posterior plaques (P<0.001) and caudal PIs (P<0.001). Greatest mid-BA angles were observed with lateral BA plaques (P=0.03) and middlelocated PIs (P=0.03).
Conclusions
Greater mid-BA angulation may enhance lateral plaque formation, causing lateral and middle PIs, whereas greater BA-VA angulation may enhance posterior plaque formation, causing paramedian or caudal PIs.
Introduction
Pontine infarctions (PIs) present diverse clinical syndromes by location [1]. Caudal PIs are more frequently present as severe unilateral weakness, whereas rostral PIs tend to be associated with ataxic symptoms [2]. Small deep PIs are associated with sensory symptoms and eye movement disorders [3]. However, the reason for this difference in PI location and the factors affecting it have not been clearly verified.
Hypertension was previously considered a major factor affecting deep PI [2]. On the other hand, paramedian PIs were associated with ectasia of the basilar artery (BA), which may stretch and distort the orifices of the paramedian perforators or alter the blood flow that forms in areas prone to early atherosclerosis that is invisible on conventional neuroimaging [4]. The presence of a BA plaque in patients without a significant BA stenosis on high-resolution magnetic resonance imaging (HR-MRI) was more closely associated with paramedian PIs than deep PIs [5]. HR-MRI is sensitive for detecting early atherosclerotic changes, and the location of these early atherosclerotic plaques is likely associated with artery shape, which influences the hemodynamic properties and atherosclerosis development [6].
The posterior circulation has a greater degree of geometric variation than the anterior circulation. The vascular geometry of the posterior circulation may include dominance of the vertebral arteries (VAs), course of the BA, and angles of the VAs and BA. PI type and vertical location may be influenced by the location of the BA plaque, which is likely associated with the vascular geometry, especially the angles of the vertebrobasilar arteries. Here we hypothesized that the angles of the vertebrobasilar system may affect the BA plaque location in isolated PI patients, whereas the BA plaque location may differ by PI type and vertical location. Patients without significant BA stenosis, rather than those with significant stenosis, would be optimal subjects for exploring the relationship between vertebrobasilar system angles and the early atherosclerotic changes influencing PI location.
Methods
Participants
Patients with a PI within 7 days of stroke onset, who were admitted to the stroke center of Kyung Hee University Hospital between July 2011 and March 2016, were screened. Among them, patients without BA stenosis from the time-of-flight magnetic resonance angiography (TOF-MRA) were consecutively enrolled. PI presence and location were confirmed using diffusion-weighted imaging (DWI) and fluid-attenuated inversion recovery (FLAIR) images taken on the day of admission. At the same time, TOF-MRA was performed and all patients with an isolated PI without BA stenosis underwent an additional HR-MRI based on the DWI and TOF-MRA findings. Patients with (1) an embolic source in the proximal artery or heart and (2) those showing poor image quality were excluded. Stroke severity and functional outcome at discharge were measured using National Institute of Health Stroke Scale (NIHSS) score and modified Rankin Scale (mRS) score. Institutional Review Board of Kyung Hee University Hospital (KMC IRB 2016-06-201) approved this study but waived the need for informed consent because of its retrospective nature and minimal risk to patients.
Imaging acquisition
MRI was performed using a 3.0 T Philips scanner (Philips Healthcare, Eindhoven, the Netherlands) with a standard 16-channel neurovascular coil on the day of admission. The sequences included DWI, FLAIR, enhanced T1 weighted image, gradient echo image, TOF-MRA, and proton density black blood image (PD-BBI). PD-BBI was obtained using the following parameters along the BA axis: relaxation time/echo time=2,500/30 ms, matrix=320×220, field-of-view=120×110 mm, slice thickness/gap=2/0 mm, longitudinal coverage=60 mm (30 slices), actual voxel size=0.375×0.5×2 mm, and reconstructed voxel size=0.23×0.23×2 mm. The pre-regional 80-mm-thick saturation pulse was used to saturate the incoming arterial flow.
PI and basilar plaque
PI location was classified by type and vertical location (Figure 1). PI type was classified as (1) paramedian PI (when the ischemic lesion abutted the base of the pons); (2) deep PI (when the ischemic lesion did not reach the base of the pons); or (3) lateral PI (when the lesion was located lateral to the pons). Vertically, the PI was classified as: (1) rostral (at the level of a relatively round shape with a small round aqueduct); (2) middle (at the level of a square fourth ventricle, large middle cerebellar peduncles, and silhouettes of the trigeminal nerves); and (3) caudal (at the level of a similarly shaped pons to the middle pons level but with images of the facial/acoustic nerves and grooves instead of the trigeminal nerves) PI according to the shape of the pons and the fourth ventricle [2].
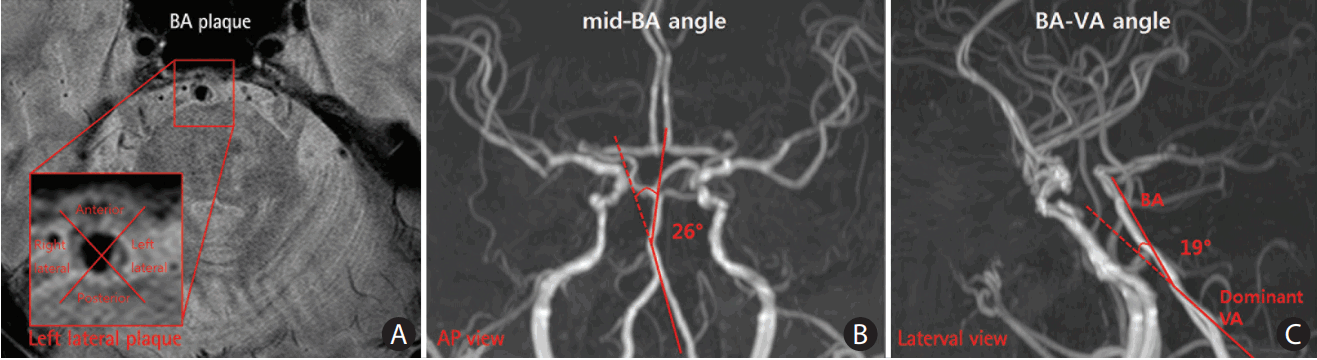
BA plaque presence, location, and length were assessed on the PD-BBI. A focal and eccentric wall thickening on the PD-BBI of BA was defined as a BA plaque [5]. The culprit plaque for the PI was defined as the plaque observed inside the BA from the identical axial slice where the PI was observed. The BA was divided into four quadrants to determine the plaque location (Figure 2A): anterior, posterior, or lateral (right and left). If the plaque was observed in more than two quadrants, the quadrant with the maximal BA plaque was chosen. Since the slice thickness of the MRI protocol was 2 mm, the plaque length was calculated as being twice the number of axial slices with a visible basilar plaque culprit for the PI.
Vertebrobasilar artery angles
The VA and BA angles were measured at the anteroposterior (AP) view and the lateral view of the 3D-reconstructed TOF-MRA (Figure 2). The VA dominance and mid-BA angle were determined and measured in the AP view. The angle between the dominant VA and BA was measured in the lateral view. The dominant VA was defined as the VA with a larger diameter or connected to the BA with a straighter manner [7]. Imaginary lines were drawn from the mid-BA to the vertebrobasilar junction and the top of the BA in the AP view (Figure 2B). The maximum angle between these two imaginary lines was considered the mid-BA angle. In the same manner, imaginary lines were drawn from the vertebrobasilar junction to the BA and the dominant VA, whereas the angle between the two lines was considered the lateral BA-VA angle (Figure 2C).
Statistical analysis
First, demographics, risk factors for atherosclerosis, and vascular geometry were compared between patients with and those without BA plaques. Multivariate analysis was performed to explore the independent factors associated with the presence of BA plaques. Demographic data, conventional risk factors for atherosclerosis, and angles of the vertebrobasilar arteries were selected for entry into the model. Second, demographics, risk factors, and vascular geometry were compared among patients with paramedian, deep, and lateral PIs. Third, the identical comparison was performed among patients with rostral, middle, and caudal PIs. The chi-square test, Fisher exact test, oneway analysis of variance, or Student t-test was appropriately used to compare the variables. P-values <0.05 were considered statistically significant. SPSS version 18 (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis.
Results
During the study period, 102 patients with isolated PIs who underwent HR-MRI were enrolled. Six patients were excluded (two with atrial fibrillation and four with significant stenosis at the proximal VA); ultimately, 96 were included in the analysis. The mean age was 69±11 years, and 49 patients (51%) were male. PIs were classified as paramedian in 70 (72.9%), deep in 17 (17.7%), and lateral in nine patients (9.4%). Fifty-one patients (53.1%) had a right PI, 44 (45.8%) had a left PI, and one patient (1.0%) had bilateral PI. Vertically, the PIs were located at the rostral (32.3%, n=31), middle (42.7%, n=41), and caudal (25.0%, n=24) regions.
BA plaques were observed in 64 patients (66.7%) on HR-MRI. There was no difference in risk factors between patients with and those without a BA plaque (Table 1). Plaques were more frequently observed in patients with paramedian or lateral PIs than those with deep PIs (72.9% or 77.8% vs. 35.3%, respectively; P=0.01). Patients with a BA plaque showed a higher discharge NIHSS score (4 [interquartile range (IQR), 3 to 5] vs. 2 [IQR, 1 to 4], P=0.006) and mRS score (2 [IQR, 2 to 3] vs. 1 [IQR, 1 to 3], P=0.007) than those without a plaque. The BA-VA angle was greater in patients with a BA plaque than in those without (35˚±13˚ vs. 23˚±13˚, P<0.001) (Table 1). After adjusting for covariates, old age (odds ratio [OR], 1.071; 95% CI, 1.020 to 1.123; P=0.006) and a large BA-VA angle (OR, 1.077; 95% CI, 1.033 to 1.124; P=0.001) were independently associated with the presence of a BA plaque.
Plaque location by PI type
BA plaques were most frequently located at the posterior wall (46.9%, n=30), followed by the lateral (32.8%, n=21) and anterior (20.3%, n=13) walls of the BA. Of the 21 lateral wall plaques, 15 were located at the ipsi-lesional side of the PI, whereas six were located at the contra-lesional side.
There was no difference in risk factors by PI types (Table 2). BA plaque location differed by PI types (P=0.03); posterior wall plaques were more frequent in patients with paramedian PIs than in those with deep or lateral PIs (37.1% vs. 11.8% or 22.2%, respectively). Lateral wall plaques were more frequent in patients with lateral PIs than in those with paramedian or deep PIs (55.5% vs. 20.0% or 11.8%, respectively). The mid-BA angle also differed by PI types (P=0.009); patients with a lateral PI had a greater mid-BA angle than those with a paramedian or deep PI (36º±23º vs. 21º±16˚ or 31º±18˚, respectively).
Plaque location by PI vertical location
There was no difference in risk factors by PI vertical locations (Table 3). However, the BA plaque location differed by PI vertical location (P<0.001); BA plaques were observed in a high proportion of patients with middle (75.6%) and caudal (70.8%) PIs. BA plaques were frequently located at the lateral (34.1%, n=14) and anterior (26.5%, n=11) wall in patients with middle PIs and at the posterior wall (58.3%, n=14) in patients with caudal PIs. Only 51.6% of patients with a rostral PI had a BA plaque (Table 3).
The BA-VA and mid-BA angles also differed by BA plaque vertical locations; the BA-VA angle was greater in patients with caudal PIs than in those with rostral or middle PIs (41˚±13˚ vs. 28˚±13˚ or 27˚±13˚, respectively; P<0.001). The mid-BA angle was greater in patients with middle PIs than in those with rostral or caudal PIs (29˚±18˚ vs. 21˚±19˚ or 18˚±13˚, respectively; P<0.001).
Plaque location and vertebrobasilar artery angles
There was no difference in risk factors except age among patients with different BA plaque locations (Table 4). However, the BA-VA angle differed by BA plaque location; the BA-VA angle was greater in patients with posterior plaques than in those without a plaque or those with anterior or lateral plaques (38˚±14˚ vs. 23˚±13˚, 32˚±14˚, 31˚±11˚, respectively; P=0.004). The mid-BA angle differed by BA plaque locations; the mid-BA angle was greater in patients with lateral plaques than in patients without a plaque or those with anterior or posterior plaques (34˚±18˚ vs. 23˚±19˚, 23˚±13˚ or 19˚±16˚, respectively; P=0.03).
Discussion
In the present study, vertebrobasilar system angles were associated with BA plaque location, which differed according to PI types and vertical locations. BA plaques were less frequently observed in patients with a deep PI by type and a rostral PI by vertical location. High angulation of the mid-BA on the AP view was associated with lateral BA plaques, lateral PI with type, and middle PI by vertical location. A greater angle between the dominant VA and BA on the lateral view was associated with a posterior BA plaque, with paramedian PI by type, and with lower PI by vertical location.
Vascular geometry of the parental artery affects the hemodynamics inside the vasculature and likely determines preclinical atherosclerosis location [6]. Furthermore, the angulation of the parental artery might be associated with infarction presence and location [7,8]. In the vertebrobasilar system, turbulence occurs at the vertebrobasilar junction, where the two VA flows conjoin, and at the mid-BA when the flow bends because of the mid-BA angle [9,10]. However, the blood flow recovers to laminar flow at the distal BA, which may partially explain the low incidence of BA plaques in the rostral PI compared to middle and caudal PIs [9] (Supplementary Figure 1). According to our study, patients with a lateral plaque demonstrated the highest mid-BA angle from the AP view and patients with a posterior plaque demonstrated the largest BA-VA angle from the lateral view. Furthermore, the mid-BA angle from the AP view was greater in patients with middle and lateral PIs, whereas the BA-VA angle from the lateral view was greater in patients with caudal PIs. Therefore, vertebrobasilar artery angle may influence BA plaque development and location.
The presence of a BA plaque in those with an isolated PI was 66.7%, a finding that is compatible with the previous report [5]. In our study, BA plaque location differed by PI types and vertical locations. The majority of deep PIs showed no BA plaques, which suggests that small vessel disease in the perforators is a dominant stroke mechanism in deep PI [11]. On the other hand, early atherosclerotic changes in a BA might be the dominant mechanism in cases of paramedian or lateral PIs. Patients with lateral PIs showed more plaques at the lateral wall of the BA, where the lateral circumferential branches of BA perforators originate [12]. Likewise, paramedian infarctions were more likely to have plaques at the posterior BA wall, where the paramedian perforator branches originate. By vertical location, lateral BA plaques were more frequently seen in middle PIs, whereas posterior wall BA plaques were more frequently seen in caudal PIs. Patients with a posterior circulation infarction due to a significant BA stenosis previously showed predominance in BA plaque location at the anterior wall of the BA [13]. In contrast, our study demonstrated that BA plaques in isolated PIs without a BA stenosis were more frequently located at the posterior wall. Since isolated PIs without a BA stenosis are directly caused by obliteration of the perforators, which usually arise from the posterior aspect of the BA, the predominance in plaque location corresponds well with the pathophysiology of isolated PIs without BA stenosis.
Our study has several noteworthy limitations. First, the sample size was small and only a single center was included. Second, the angle was measured from the 2D plane of a 3D-reconstructed TOF-MRA (AP or lateral view). However, this method is considered valid for accurately measuring cerebral artery geometry [14,15]. Third, PD-BBI was used to evaluate BA plaques; therefore, the composition of the BA plaques could not be assessed. Finally, we measured the vascular geometry but could not evaluate the true hemodynamics that might explain the association between vascular geometry and atherosclerotic plaque seen on HR-MRI. Further studies measuring the hemodynamic factors may elucidate the exact hemodynamic mechanism linking arterial angles to BA plaque and PI locations.
Conclusions
In conclusion, a greater angulation between the dominant VA and BA from the lateral view may enhance BA plaque formation on the posterior wall at the lower pontine level and cause paramedian or caudal PIs. A greater angulation at the mid-BA from the AP view may enhance BA plaque formation on the lateral wall at the middle pontine level and cause a lateral or middle PI. A BA plaque at a specific location, determined using vascular geometry, obliterates the orifice of the perforators and affects PI type and location.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2017.00829.
Real-time flow measurement of a patient with rostral pontine infarction. Flow pattern of distal basilar artery recovers to laminar flow pattern, whereas turbulence is observed from the middle and proximal basilar artery.
Notes
Disclosure
The authors have no financial conflicts of interest.
Acknowledgements
This work was supported by a grant from Kyung Hee University in 2017 (KHU-20170850).