Updates on Prevention of Hemorrhagic and Lacunar Strokes
Article information
Abstract
Intracerebral hemorrhage (ICH) and lacunar infarction (LI) are the major acute clinical manifestations of cerebral small vessel diseases (cSVDs). Hypertensive small vessel disease, cerebral amyloid angiopathy, and hereditary causes, such as Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL), constitute the three common cSVD categories. Diagnosing the underlying vascular pathology in these patients is important because the risk and types of recurrent strokes show significant differences. Recent advances in our understanding of the cSVD-related radiological markers have improved our ability to stratify ICH risk in individual patients, which helps guide antithrombotic decisions. There are general good-practice measures for stroke prevention in patients with cSVD, such as optimal blood pressure and glycemic control, while individualized measures tailored for particular patients are often needed. Antithrombotic combinations and anticoagulants should be avoided in cSVD treatment, as they increase the risk of potentially fatal ICH without necessarily lowering LI risk in these patients. Even when indicated for a concurrent pathology, such as nonvalvular atrial fibrillation, nonpharmacological approaches should be considered in the presence of cSVD. More data are emerging regarding the presentation, clinical course, and diagnostic markers of hereditary cSVD, allowing accurate diagnosis, and therefore, guiding management of symptomatic patients. When suspicion for asymptomatic hereditary cSVD exists, the pros and cons of prescribing genetic testing should be discussed in detail in the absence of any curative treatment. Recent data regarding diagnosis, risk stratification, and specific preventive approaches for both sporadic and hereditary cSVDs are discussed in this review article.
Introduction
Cerebral small vessel diseases (cSVDs)—pathologies in small superficial and deep perforating arteries and arterioles of the brain—result in approximately one-fourth of all acute ischemic strokes, mainly in the form of lacunar infarction (LI). The Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria defines the term LI, in relation to cSVD, as the combination of (1) presentation with one of the traditional clinical lacunar syndromes, (2) normal computed tomography/magnetic resonance imaging (MRI) results or demonstration of a relevant brain stem or subcortical lesion with a diameter <1.5 cm, and (3) exclusion of an alternate etiology, such as cardiac sources of embolism or large vessel disease [1].
Intracerebral hemorrhages (ICH) also constitute a major manifestation of cSVD [2]. The majority of non-traumatic ICHs (primary ICH) originate from the spontaneous rupture of small vessels damaged by chronic hypertension or amyloid angiopathy [3]. ICH is well-known for its high overall stroke mortality and disability [4]. It carries a 1-year fatality of >50%, with only 20% of patients being independent at 6 months [4]. The burden of lacunes, with other manifestations of cSVD, are a risk factor for disability, increased mortality, and unfavorable stroke outcomes [5]. In addition to the ischemic and hemorrhagic strokes, cognitive impairment and gait disorders are frequently seen in older adults with underlying cSVD [2,6,7], causing substantial disability. It is noteworthy that the epidemiology of cSVD might differ between Asian and Western populations [8,9], and the impact of cSVD may be even larger in non-Caucasian populations [10-12]. In this review article, we aim to summarize recent advances in the prevention of primary ICH and LI, as patients with cSVDs are at risk for both conditions. We discuss the latest advances in diagnosis and management with emphasis on antithrombotic use/avoidance and individualized vascular risk factor control.
Cerebral small vessel diseases: major etiology of lacunar infarction and intracerebral hemorrhages
cSVD includes a group of vascular pathologies with various etiologies that affect the small arteries, arterioles, venules, and capillaries of the brain, leading to both ischemic and hemorrhagic consequences [2]. There are three common categories of vascular pathologies underlying cSVD.
Hypertensive small vessel disease
LI, and hypertensive ICH, mostly located in the brain’s deep grey structure, such as the basal ganglia and thalamus, are mostly related to hypertensive (HTN)-SVD (Figure 1). The underlying small vessel pathologies are mainly characterized by arteriolosclerosis, most commonly in the form of fibrinoid necrosis and lipohyalinosis, and involvement of deep-seated vessels that stem directly from the large vessels as arterial perforators [2]. Degenerative changes in the walls of the cerebral small vessels could cause ischemic LIs, microaneurysm formation, and ICH. Other commonly coexisting systemic vascular risk factors, such as abnormal glucose metabolism, may also lead to impaired endothelial function and subsequently cSVD [13]. However, the relationship between hyperlipidemia and cSVD is contradictory. A low low-density lipoprotein cholesterol (LDL-C) level has been proposed as the potentially underlying mechanism for fragile endothelium, causing higher risks of vessel rupture and ICH [14]. HTN-SVD has been associated with various additional radiological findings, including deep cerebral microbleeds (CMBs) (Figure 1B), white matter disease (leukoaraiosis) (Figure 1C), and MRI-visible enlarged perivascular spaces (EPVS) (Figure 1F) in the basal ganglia [15,16]. Recently, mixed-ICH, which refers to combined deep and lobar hemorrhage, is suggested to be associated with vascular risk factors similar to hypertensive ICH, but demonstrates more severe parenchymal damage and higher ICH recurrence risk (Figure 1D) [17,18].
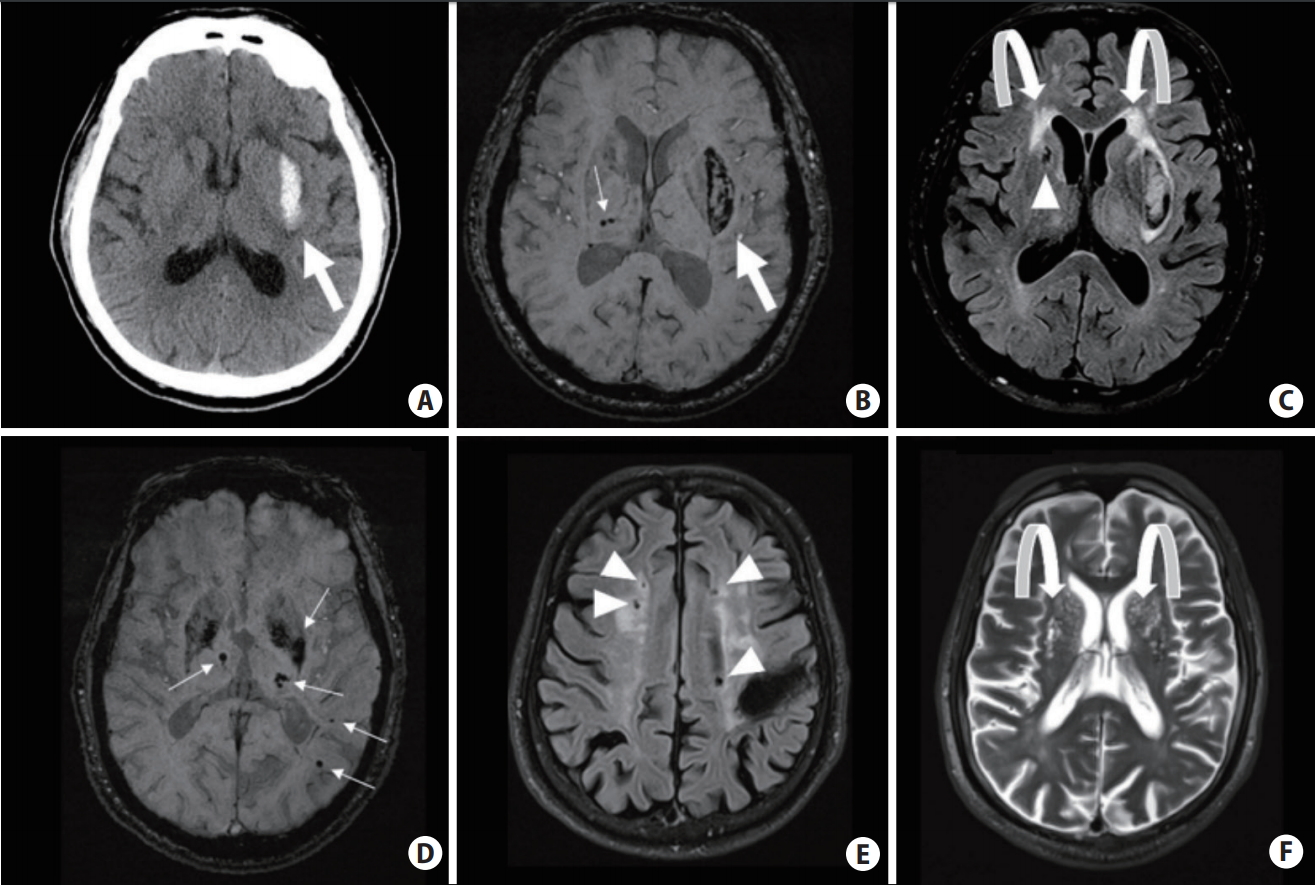
Computed tomography and magnetic resonance imaging (MRI) scans of hypertensive small vessel disease and associated bleeding risks. (A–C) Hypertensive intracerebral hemorrhage (ICH) in the left basal ganglia (big arrows); ~1.6% to 2% annual recurrence risk. Several deep microbleeds (MBs) (small arrows), periventricular white matter hyperintensities (curved arrows) and a deep lacune (arrowhead) are also seen. (D–F) Mixed-location of ICH and MBs (small arrows), associated with ~5.1% annual risk of ICH recurrence. There are several lacunes (arrowheads) and MRI-visible enlarged perivascular spaces near the basal ganglia (curved arrows) in the same patient.
Cerebral amyloid angiopathy
Cerebral amyloid angiopathy (CAA) is characterized by the progressive accumulation of β-amyloid in the leptomeningeal and cortical vessels of the brain (Figure 2) [19]. Lobar ICH is the typical presentation of CAA-related vasculopathy. The association between hemorrhagic cSVD markers and CAA is well-recognized, including strictly lobar CMB (Figure 2B) and cortical superficial siderosis (cSS) (Figure 2D) [20]. The combination of at least one lobar ICH and either one or more strictly cortical/lobar CMB or cSS defines CAA in a patient older than 55 years old when other potential causes are ruled out [21,22]. While most research has focused on its hemorrhagic manifestations, findings from radiologic and pathologic studies suggest that ischemic lesions from CAA also have substantial relevance [23]. Recently, lacunes located in the centrum semiovale (CSO) or lobar regions, collectively called lobar lacunes (Figure 2E), were reported to be a potential CAA marker [24], further expanding the etiology of LI from chronic hypertension to a different vascular pathology [25].
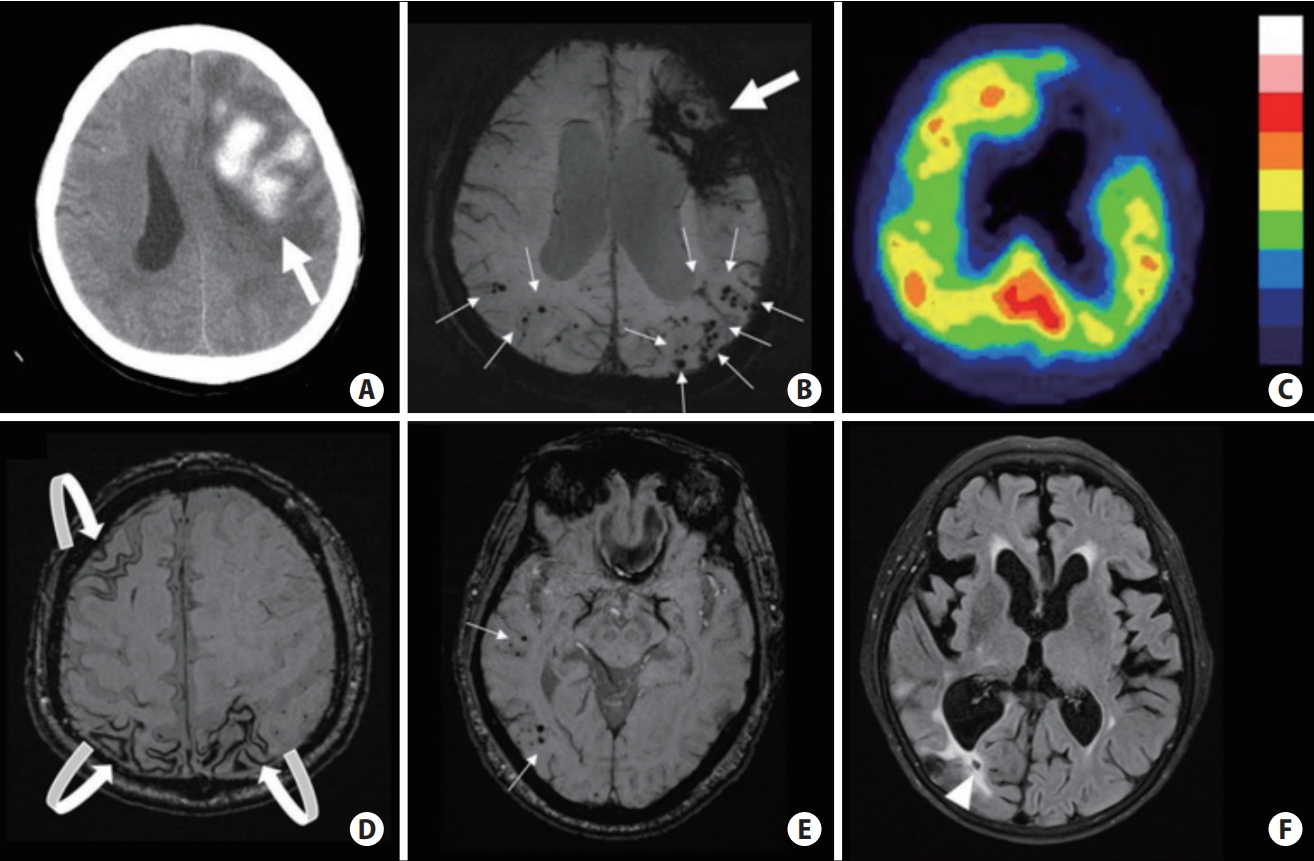
Computed tomography and magnetic resonance imaging scans of cerebral amyloid angiopathy (CAA) related pathologies and associated bleeding risk. (A, B) Probable CAA (per Boston criteria) with lobar intracerebral hemorrhage (ICH) (big arrows) and multiple cortical microbleeds (MBs) (small arrows); ~10% annual ICH recurrence risk. (C) Increased tracer uptake detected on 11C-Pittsburgh Compound B positron emission tomography scans in the same patient as A and B note the posterior predominance typical of CAA. (D) Multifocal cortical superficial siderosis (curved arrows) in a probable CAA patient who had ICH (not shown on this cut); ~27% annual risk of recurrent ICH. (E) CAA presenting with only cortical MBs (small arrows); ~5% yearly risk of first-time symptomatic ICH. (F) Lobar lacune (arrowhead) in a probable CAA patient who had ICH (not shown on this cut).
Hereditary causes of cerebral small vessel diseases
A small, but significant proportion of small vessel diseases (SVDs) are of Mendelian inheritance (Figure 3). However, it is generally considered that the currently-identified monogenic causes represent less than 20% of all monogenic forms of cSVD [26]. Among these, Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) is the most frequent and most well-known disorder, with hundreds to thousands of families diagnosed worldwide [27]. CADASIL is linked to stereotyped mutations of the neurogenic locus notch homolog protein 3 (NOTCH3) gene in chromosome 19 [28]. The course of the disorder is now well known in its common form and rarer presentations are currently being recognized. MRI white matter hyperintensities (WMH) appear often before 30 years, and MRI results are usually abnormal at age 40. In their forties or fifties, CADASIL patients present their first ischemic strokes related to small subcortical infarcts. However, approximately 30% of patients never suffer strokes [29]. The number of strokes does not seem to markedly influence the disease course, which is generally more severe in men, for yet unknown reasons [30]. The one modifiable risk factor is smoking, which multiplies ischemic stroke risk by a factor of three in these patients [31]. While it was originally thought that most patients become demented and bedridden in their sixties, benign courses have been determined, patients (particularly women) in their seventies can be seen without any symptom [29].
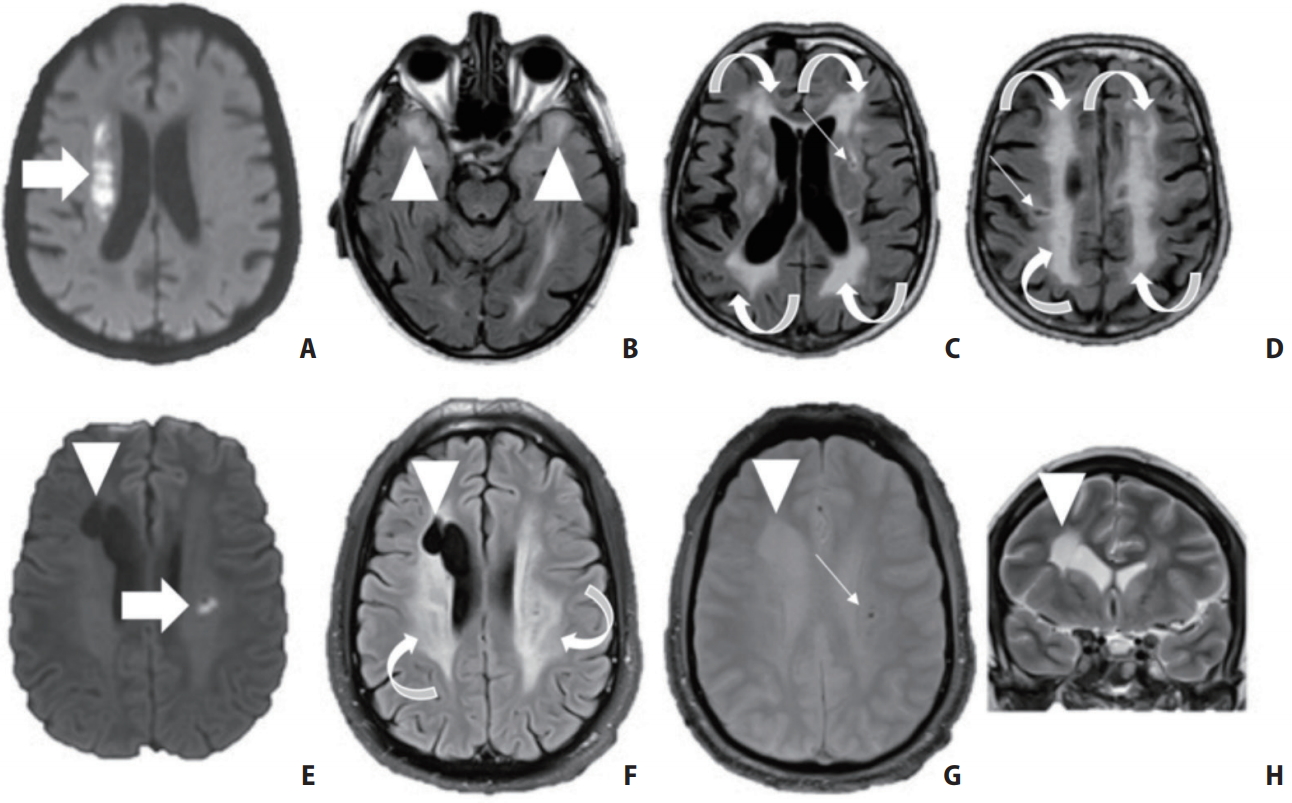
Neuroimaging characteristics of two patients with hereditary small vessel diseases (SVD). Top row: A 64-year-old male patient without medical history presented with acute left hemiplegia. (A) Imaging revealed an acute right deep-seated infarct (arrow) as well as (B, C) widespread white matter hyperintensities (curved arrows, arrowheads) and (C, D) multiple lacunes (small arrows). (B) Fluid attenuated inversion recovery (FLAIR) also shows bilateral anterior temporal white matter hyperintensities (arrowheads). The patient was diagnosed with Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) based on genetic testing. Bottom row: a 32-year-old male patient presented with acute dysarthria related to a small subcortical infarct (E, arrow). (F) Brain MRI showed otherwise diffuse white matter hyperintensities (curved arrows), (E–H) a right porencephalic lesion (arrowhead) and (G) microbleeds (small arrow). The patient was proven to have a COLIVA mutation.
The second most frequent cause of hereditary cSVD is related to dominant HTRA1 mutations in chromosome 10, which are estimated to represent approximately 5% of hereditary cSVD [26,27]. Given the recent characterization of its role in a form of dominantly inherited SVD, prospective data are lacking, thus far, to identify the disease characteristics. Most reported patients presented with severe SVD in the elderly, sometimes in a context of uncontrolled hypertension. The pattern of WMH does not seem to be specific [26].
Autosomal recessive forms related to mutations in the same gene led to the extremely rare Cerebral Autosomal Recessive Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CARASIL) disorder, diagnosed so far in a few tens of individuals, particularly in Asians [32] and reports on Caucasian patients are exceptional [33]. This disorder is characterized by spondylosis and alopecia in most cases, and most reports emphasize the severity of the disorder, often leading to disability, dementia, or death as soon as the fifth decade [34].
The third most frequent form is related to autosomal dominant mutations in COLIVA1 or COLIVA2 genes, leading to different phenotypes that mostly predispose to ICH [35]. Many unknowns remain as illustrated by the presumed high frequency of mutation careers. The disease includes various extra-neurological features, including ocular, renal, and muscular manifestations.
A recently recognized phenotype corresponds to mutations of the promotor area of the COLIVA1 gene and lead to the pontine autosomal dominant microangiopathy and leukoencephalopathy (PADMAL) phenotype. In this rare disorder, small subcortical ischemic lesions and lacunes accumulate within the pons, with a clear predominance with respect to other brain regions [36]. Other genes have been described, but the associated disorders are rarely encountered in clinical practice. In line with CADASIL and CARASIL, the acronym CARASAL has been proposed to describe a rare disorder related to autosomal mutations of the cathepsin A gene [37]. Other forms, such as familial amyloid angiopathy, are also rarely seen. In comparison to sporadic forms, and in addition to familial history of CAA, the presence of calcifications of the cerebral cortex is quite specific [38]. Hereditary forms of CAA cause even more frequent recurrent lobar ICHs starting in the 30s to 40s and cause structural brain injury in mutation careers even before the first ICH [39,40].
The diagnosis of cSVD is mainly based on lesions in the brain parenchyma mostly located in the subcortical structures. These lesions can be readily detected on MRI, including intraparenchymal hemorrhage, CMB, cSS, lacunes, WMH, and MRI-visible EPVS. The differentiation between HTN-SVD and CAA is traditionally based on hemorrhage location. CAA-related ICH and CMB are located exclusively in the cortical/lobar regions, while hypertensive emorrhage is usually located in the deep parts of the brain [3,21,41]. The presence of strictly lobar ICH/CMBs showed 100% accuracy for CAA in a radiologic/pathologic validation study [21]. A cSS is another hemorrhagic feature of CAA and is typically absent in other types of cSVD [20,22,42]. Similar to the distribution of hemorrhagic lesions, the topography of lacunes was shown in a recent study to be different between CAA and HTN-SVD, with lobar (or non-deep seated) lacunes being associated with CAA while deep lacunes were found to be more frequent with HTN-SVD [24]. White matter disease is prominent in both HTN-SVD and CAA [43-48]. Different WMH patterns between HTN-SVD and CAA have been proposed, but the significant overlap in WMH patterns suggests that leukoaraiosis is probably multifactorial in many cases [45]. MRI-visible EPVS, which might imply cerebrospinal fluid (CSF) drainage impairment, has also been suggestive of an important marker in cSVD. Previous studies have showed that severe EPVS in the basal ganglia was associated with HTN-SVD, while high-degree EPVS in CSO was linked to CAA [16,49].
In addition to radiological markers, a decreased amyloid β (Aβ) level in the CSF, particularly Aβ40 and Aβ42, may serve as a molecular biomarker to identify CAA patients [50]. Another important advancement is the application of amyloid positron emission tomography (PET) using 11C-Pittsburgh Compound B or 18F-Florbetapir [6,51]. Amyloid PET provides a noninvasive method to directly quantify β-amyloid presence and burden in vivo (Figure 2C). Given that it labels both cerebrovascular and parenchymal amyloid, amyloid-PET appears to have better diagnostic accuracy under appropriate clinical settings, such as in differentiating patients with probable CAA from cognitively normal healthy controls or patients with deep ICH [51].
The identification of hereditary causes is of high interest in patients with a history of stroke. First, when natural history is well described, there is an opportunity to inform patients, given that the fate of the disease might be different from that of sporadic forms. Currently, this applies mostly to CADASIL, for which benign forms have been regularly observed. In addition, modifiable factors may be known to influence the disease course and controlling such risks is important. For instance, smoking is strongly associated with stroke recurrence in CADASIL, while it is clearly known that even minor head trauma can trigger devastating ICH in COLIVA1 patients. The asymptomatic forms present unique challenges. In the absence of any curative treatment, the pros and cons of prescribing a genetic test should be discussed with the patients. In a major center dealing with a high volume of hereditary cSVD such as Lariboisière Hospital, asymptomatic individuals must undergo a multi-professional consultation before genetic testing can be performed [52].
By contrast to what was previously thought, it seems that no aspect of imaging is specific to any hereditary etiology. For long, it has been considered that anterior temporal pole hyperintense lesions, as well as that of the superior frontal gyrus and subinsular areas, were specific to CADASIL [53], but these aspects might be also observed in sporadic forms. It is possible that they are more frequent in hereditary causes including NOTCH3 negative forms. Other aspects might be more often observed in some cases. For instance, an état criblée of the basal ganglia may be more prevalent in HTRA1 autosomal dominant related forms [26]. In addition, multiple small infarcts and lacunes of the pons, contrasting with a relative preservation of other brain areas, is in favor of mutations of the promotor of COLIVA1 (the PADMAL phenotype) [36]. However, given the diversity of clinicoradiological presentations of cSVD, even within families affected by the same disorder, none of these aspects are very helpful. By contrast, a discrepancy between a low risk factor profile and a severe clinicoradiological presentation is helpful, particularly when familial history is lacking.
Diagnosing the underlying vascular pathology is crucial because there is a significantly different risk of recurrent ICH between CAA and HTN-SVD (Figures 1 and 2). Patients who suffer from CAA-related ICH carry around 10% of an annual ICH recurrence risk. Such risk is even higher if cSS is present, and increases up to 27% annually with widespread cSS [20,54]. Patients with multiple strictly lobar CMB (without associative ICH) had risk factors and neuroimaging profiles similar to CAA, and possess a 5% annual risk of developing first-time symptomatic ICH during follow-up [54]. In contrast, the ICH risk is reported as 2% per year in survivors of hypertensive ICH [17,55]. In patients with mixed-ICH (Figure 1E), which may represent the consequence of more severe underlying HTN-SVD, the ICH recurrence rate increases to 5.1% per year according to a recent study [17]. Thus, identifying the underlying microangiopathy is crucial, especially in situations where patients have an indication for antithrombotic use, to balance hemorrhagic versus ischemic risks. The diagnosis may further influence clinical decisions regarding management of vascular risk factors, especially considering statin use for hyperlipidemia [14].
The ICH risk seems very low in Caucasian patients with CADASIL. Among 278 CADASIL patients from the Paris-Munich cohort followed for 3 years, 55 strokes occurred and all were ischemic; no ICH was observed [31]. Even more recently, 69 ischemic strokes were observed but no ICH among 369 patients followed for a median of 3.9 years, leading to an estimated average incidence of ischemic stroke of 4.4 per 100 people per year [56]. The ischemic stroke risk was higher in patients with microbleeds than in those without, but all predictors were otherwise equal [56]. While rare ICH in hereditary cSVD has been clearly documented in Western countries, ICH risk might be far larger in Asian populations. In a cohort of 94 CADASIL patients from Korea, 16 had an ICH [57]. ICH risk seems to be strongly linked to hypertension [58], which might be more prevalent in Asian populations. To our knowledge, there is no data on estimations of ICH risk or ischemic stroke recurrence in patients with COLIVA1 mutations or other rare hereditary cSVD.
In summary, patients who have any combination of these vascular pathologies or associated parenchymal lesions are at risk for both ischemic and hemorrhagic stroke. Recent improvements in understanding the above-mentioned radiological markers for sporadic cSVD can help the clinician stratify ICH risk in individual patients. There are general good practice measures for stroke prevention in patients with cSVD, while individualized measures tailored for each patient are vital. These approaches will be further discussed in the subsequent sessions.
Use or avoidance of antithrombotics for prevention of ICH and LIs
Recurrent strokes in patients with previous LI or ICH are common. As discussed above, some ICH survivors might have ischemic strokes, and those with LI have an increased risk for recurrent ICH [59-61]. Although antithrombotics play an important role in ischemic stroke prevention, determining the appropriate secondary prevention after LI is a therapeutic dilemma, especially knowing that ICH frequently causes devastating outcomes and is the most feared complication of antithrombotics. Clinical decision making should always be based on the assessment of ischemic stroke reduction weighed against hemorrhagic risks associated with antithrombotic use in patients with underlying cSVD. Oral anticoagulants are not considered for cSVD-related stroke prevention, including recurrent LIs, as they disproportionately increase ICH risk. Patients with hemorrhagic cSVD markers are at a particularly high risk for developing ICH under anticoagulation, and the use of such medications should be prevented, even when indicated for a concurrent pathology when alternatives exist, such as left atrial appendage closure in non-valvular atrial fibrillation [55,62,63].
Although studies focusing on LI are rare, the benefits of aspirin or other antiplatelets are considered to be similar between lacunar and non-LIs based on subgroup analyses of randomized controlled trials (RCTs) in all types of ischemic strokes [64]. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH) trial revealed higher incidences of major bleeding in patients with long-term dual antiplatelet therapy (75 mg clopidogrel and 75 mg aspirin combination vs. 75 mg clopidogrel) [65]. The Secondary Prevention of Small Subcortical Strokes (SPS3) trial is the only study focusing on the efficacy of antiplatelet agents in stroke prevention for patients with LI [60]. This study was a RCT that included 3,020 patients who were randomized to receive aspirin monotherapy (325 mg aspirin) or dual antiplatelet therapy (325 mg aspirin and 75 mg clopidogrel). Dual antiplatelet therapy was found to increase the incidence of major hemorrhage and mortality rate, and thus, the trial was stopped early. Moreover, the risk for recurrent stroke was not significantly reduced by dual antiplatelet therapy. Overall, these studies showed that dual antiplatelet therapy might increase major bleeding risk without providing additional stroke reduction benefits for LIs. Currently, anti-platelet monotherapy is recommended to prevent recurrent strokes after LI [64]. Cilostazol and triflusal, while offering similar efficacies in stroke prevention, may have lower risks of hemorrhage than aspirin in recent studies [66,67]. The subgroup analysis of cilostazol for prevention of secondary stroke (CSPS2) study in patients with LI (100 mg cilostazol twice a day vs. 81 mg aspirin once a day) showed that the incidence of ICH was significantly lower in patients using cilostazol than aspirin alone [68]. In a recent trial for the prevention of cardiovascular events in Asian patients with ischemic stroke at high risk of cerebral haemorrhage (PICASSO) [69], authors enrolled 1,534 patients with ischemic stroke and a history of or imaging findings of ICH or two or more microbleeds, and randomized them to receive either aspirin or cilostazol treatments. During a median follow-up of 1.9 years, the incidence of ICH was 0·61 per 100 people per year in patients who received cilostazol and 1.20 per 100 people per year in those who received aspirin (hazard ratio [HR], 0.51; 97.5% confidence interval [CI], 0.20 to 1.27; superiority P=0.18). Although the results are not statistically significant, they are consistent with previous reports from Asia, and suggest that cilostazol may be an antiplatelet suitable for patients with ischemic stroke at increased ICH risk. However, cilostazol research has been scarce in non-Asian patients, and further large-scale studies are warranted to validate the results in non-Asian populations [70].
cSVD patients with a concomitant cardioembolic source or other problems for whom anticoagulation would normally be used present a therapeutic dilemma. Life-long anticoagulation is contraindicated after cSVD-related ICH, and should also be avoided to the extent possible in patients with cSVD-related lacunar strokes, given that anticoagulants may lead to increased major bleeding risks in such patients [63,71-73]. All of the phase three trials of the four non-vitamin K oral anticoagulants have excluded patients with a history of ICH or those with a high risk for hemorrhage [74]. For patients without ICH that have subtle hemorrhagic lesions, such as CMB and cSS, the use of long-term anticoagulation is also problematic [63,75,76].
Nonpharmacological approaches, such as left atrial appendage closure, might provide potential alternatives to life-long anticoagulation for non-valvular atrial fibrillation and are further discussed in an accompanying review article in this issue of the Journal of Stroke as well as in a separate recent review [63,77]. In acute myocardial infarction and deep venous thrombosis, anticoagulation time should be minimized to the lowest extent possible if the patient has cSVD. A careful riskbenefit assessment is always needed in individual patients after stratifying the ICH risks (summarized in the previous parts and figures). Patients at a low ICH risk might tolerate anticoagulation for shorter timeframes [78].
Individualized optimal control of vascular risk factors
Hypertension
Hypertension is the best known and prevalent risk factor for stroke, especially for cSVD-related LI and ICH [79]. Prolonged exposure of intracranial arterioles to hypertension can be associated with endothelial dysfunction, ultimately causing cSVD [80]. Although correction of hypertension is an established way for stroke prevention, the optimum blood pressure (BP) in patients with cSVD has been unknown; decreased cerebral perfusion might be expected under low BP states if autoregulation was chronically damaged.
The perindopril protection against recurrent stroke study (PROGRESS) trial reported a reduction in BP by 9/4 mm Hg corresponding to a reduction in recurrent stroke by 28% (95% CI, 17% to 38%) compared with placebo during a mean follow-up of 3.9 years in 6,105 patients with previously symptomatic cerebrovascular disease; however, there was no data regarding patients with a qualifying event of LI [81]. In the Prevention Regimen for Effectively Avoiding Second Strokes (PRo-FESS) trial, a reduction in BP by 3.8/2.0 mm Hg corresponded to a nonsignificant reduction in recurrent stroke by 5% (95% CI, –4% to 14%) compared with the placebo during a mean follow-up of 2.5 years in 20,332 patients with recent ischemic stroke, in which 52% of the qualifying strokes were LI [82]. The SPS3 trial is the only RCT to test the target BP level for secondary prevention in patients with symptomatic LI. This study included 3,020 patients and compared two systolic BP targets of 130 to 149 mm Hg versus <130 mm Hg with a mean follow-up of 3.7 years. There was a 19% (95% CI, −3% to 36%) non-significant reduction in recurrent stroke, but the lower BP target group (<130 mm Hg) had markedly reduced ICH risks (HR, 0.37; 95% CI, 0.14 to 0.89) [83]. Thus far, there is no RCT regarding BP control for secondary prevention in ICH survivors; however, in a single-center observational study, inadequate BP control was associated with a higher lobar and non-lobar ICH recurrence risk [84]. Therefore, current evidence suggests that more stringent BP control (within or close to normal limits) should be favored over less strict control for stroke prevention in patients harboring cSVDs [85-87].
While there is no proof that BP lowering is efficient for stroke prevention in hereditary cSVD, there are indirect arguments suggesting a reduction in ICH risk with strict BP lowering in CADASIL patients. However, it is important not to lower BP excessively, and particular attention is needed for specific situations, such as general anesthesia, because some reports have clearly highlighted a risk of multiple concurrent small subcortical infarcts [88]. In COLIVA1 patients, BPs are monitored and normotension is actively targeted with antihypertensive medication use when needed.
Diabetes
Both diabetes mellitus and metabolic syndrome are related to increased incidence of ischemic stroke and ICH, and to the risk of recurrent strokes in patients with LI [89-92]. In the SPS3 trial, which specifically included patients with symptomatic LI, diabetic patients were almost twice as likely to have a recurrent, especially ischemic, stroke (HR, 1.8; 95% CI, 1.4 to 2.4). Diabetic patients also had more frequent intracranial stenosis, infarcts involving posterior circulation, and more extensive WMH [93]. Data on blood sugar control and ICH risk remains limited. In a community-based prospective cohort study in China with 96,110 patients, either low (<4.0 mmol/L or 72 mg/dL) or high (≥6.1 mmol/L or 110 mg/dL) fasting blood glucose levels led to elevated incident ICH risk in a median follow-up of 9 years [13]. Keeping patients euglycemic to the extent possible and preventing hypoglycemia might be potentially beneficial in cSVD prevention, but these approaches need to be further validated.
Hyperlipidemia
Hyperlipidemia, particularly elevated LDL-C levels, is an important risk factors for ischemic stroke and is also a general target for secondary preventive measures. However, there are concerns regarding possible associations between low lipoprotein levels and statin use on ICH risk [14]. The Stroke Prevetion by Aggressive Reduction in Cholestrol Levels (SPARCL) trial demonstrated the efficacy of lipid-lowering therapy in patients with a recent (<6 months) transient ischemic attack or stroke and a baseline cholesterol level between 100 and 190 mg/dL. In this trial, atorvastatin reduced the incidence of recurrent stroke, from 13.1% to 11.2% over a 5-year follow-up period [94]. However, patients with ICH who received statins were found to have a significantly increased ICH recurrence risk [95,96]. A post hoc analysis of the SPARCL trial shows a significantly increased ICH risk in patients with baseline cSVD-related LI who received statins (2.8% vs. 0.6%; HR, 4.99; 95% CI, 1.71 to 14.61), but also shows a counterbalancing trend toward benefit for ischemic stroke reduction (11.2% vs. 14.6%; HR, 0.76; 0.57 to 1.02) [96]. Benefits and potential risks of statin use should be explicitly discussed with all patients who might have a higher hemorrhagic risk and an algorithm for statin treatment in this context was published in a recent review article [14].
Homocysteine elevation and its treatment
Homocysteine promotes the endothelial inflammatory response and oxidative stress-induced endothelial damage, and hyperhomocysteinemia is a risk factor for cSVD [97]. The availability and the low side effect profile of a multivitamin supplementation for hyperhomocysteinemia treatment (oral folic acid, B6- and B12-vitamins), might provide a favorable addition to the standard preventive measures [98]. In the VITAmins TO Prevent Stroke (VITATOPS) trial, homocysteine-lowering therapy and B-vitamins were associated with a significant reduction in WMH volume change in those with severe baseline cSVD [99].
Renal failure, hepatic failure, and other systemic vascular risk factors
cSVD has been reported to correlate strongly with chronic kidney disease. Decreased creatinine clearance appears to be a contributing factor to silent LIs, and even possibly to other types of cSVD-related lesions, such as WMH and CMB [100]. Chronic hepatic dysfunction and other systemic diseases have also been linked to cSVD and ICH. Therefore, it is important to optimize the function of other organ systems to the extent possible to minimize cSVD risks.
Specific considerations
Amyloid lowering for CAA
Preventive treatment for CAA is currently limited, but there are growing interest and international efforts to identify potential candidates that inhibit the formation or deposition of β-amyloid. Only one phase two trial with tramiprosate, an oral agent that decreases vascular Aβ burden in the transgenic mouse models, was published and confirmed its safety [101]. Thus far, there is no specific therapy shown to lower ICH recurrence in CAA in humans.
Conclusions
LIs and intraparenchymal hemorrhages share a common etiology—the cSVDs in most cases. These conditions are mostly sporatic, but a better understanding of hereditary forms is developing, resulting in an accurate diagnosis of the latter. Several good practice measures, such as optimal BP and diabetes management, can help reduce the risk of both stroke types in patients with SVDs. Antiplatelet combinations and anticoagulants have no specific role in ischemic lacunar stroke prevention and increase the risk of usually fatal/disabling brain bleeds in these patients. A good understanding of parenchymal hemorrhage risk is crucial for appropriate management, especially for use/avoidance of antithrombotics; such risk stratification can be monitored using available MRI markers.
Notes
Disclosure
The authors have no financial conflicts of interest.
Acknowledgements
This work was made possible by grants from the National Institute of Health (M. Edip Gurol, NS083711).