Cerebral Small Vessel Disease: A Review Focusing on Pathophysiology, Biomarkers, and Machine Learning Strategies
Article information
Abstract
Cerebral small vessel disease (cSVD) has a crucial role in lacunar stroke and brain hemorrhages and is a leading cause of cognitive decline and functional loss in elderly patients. Based on underlying pathophysiology, cSVD can be subdivided into amyloidal and non-amyloidal subtypes. Genetic factors of cSVD play a pivotal role in terms of unraveling molecular mechanism. An important pathophysiological mechanism of cSVD is blood-brain barrier leakage and endothelium dysfunction which gives a clue in identification of the disease through circulating biological markers. Detection of cSVD is routinely carried out by key neuroimaging markers including white matter hyperintensities, lacunes, small subcortical infarcts, perivascular spaces, cerebral microbleeds, and brain atrophy. Application of neural networking, machine learning and deep learning in image processing have increased significantly for correct severity of cSVD. A linkage between cSVD and other neurological disorder, such as Alzheimer’s and Parkinson’s disease and non-cerebral disease, has also been investigated recently. This review draws a broad picture of cSVD, aiming to inculcate new insights into its pathogenesis and biomarkers. It also focuses on the role of deep machine strategies and other dimensions of cSVD by linking it with several cerebral and non-cerebral diseases as well as recent advances in the field to achieve sensitive detection, effective prevention and disease management.
Introduction
Cerebrovascular diseases remain a leading cause of death and functional disability worldwide. The global burden of disease 2013 study undertaken by American Heart Association identified greater stroke burden in men than women with 133/100,000 males-years and 99/100,000 females-years incidence of ischemic stroke [1]. According to Centers for Disease Control and Prevention, ischemic stroke is the most common type of stroke, contributing to almost 87% of the incidences among total stroke incidence [2].
On the basis of different clinical pictures and the characteristic appearance of the lesion and coexisting vascular diseases, ischemic stroke is subclassified into lacunar, embolic, and thrombotic cerebral infarction [3]. Cerebral small vessel disease (cSVD) is a term used for different pathological processes that affect the small vessels of the brain, including small arteries, arterioles, capillaries, and small veins. cSVD has a crucial role in lacunar cerebral infarction and deep or cortical haemorrhages [4]. In addition to cognitive decline [5] and dementia [6], gait problems [7] are also frequently associated with cSVD.
Despite advances in the last decades in the field of neuroimaging and biomarkers, the pathogenesis of vascular disease is not well known. Damage to the blood-brain barrier (BBB) seems to be a common and early mechanism in the different forms of sporadic cSVD [8]. With the discovery of several subtypes of hereditary and forms different genetic, molecular, and cellular disease mechanisms has expanded the knowledge about the pathophysiology of this disease. We have thus divided the cSVD based upon two different aspects: (1) pathological cSVD and (2) genetic cSVD.
This review gives a comprehensive picture of small vessel disease (SVD) covering its pathological subtypes in sporadic and hereditary forms with special emphasis in the role of the BBB dysfunction in the initial pathogenesis. Moreover a complete description of the imaging markers, the development of automated methods for its detection and quantification and some unraveling relation to non-cerebral components is presented. Altogether, this review provides researchers to generate an in-depth understanding of cSVD, it will further aim towards exploring its prevention and treatment strategy.
Types of cerebral small vessel disease
The term cSVD is used with various meanings in different contexts. The topography of the underlying microvascular pathology is different in each case of cSVD. Additionally, to elucidate cellular and molecular mechanisms of hereditary forms of cSVD, it is important to understand genetics behind cSVD pathology. To describe a range of genetical and pathological features associated with cSVD, it is stratified in different subtypes here taking these two attributes (pathology and genetics).
Pathological subtypes of sporadic cerebral small vessel disease
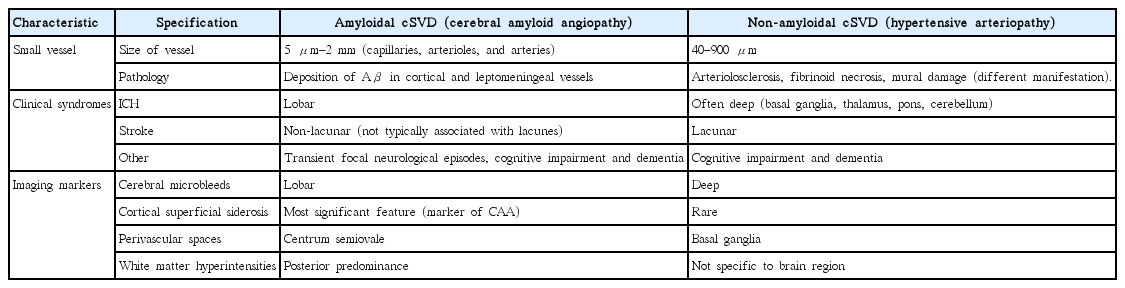
Approximately one-fifth of symptomatic strokes are lacunar stroke syndromes [9], which are often the more severe (sometimes lethal) kind of strokes namely spontaneous parenchymal brain hemorrhage (PBH). These are associated with cSVD [10]. However, even though sporadic cSVD is the leading cause of PBH, the topography of the underlying microvascular pathology is different in each case. To provide some kind of a general framework, cSVD is categorized in two main forms. The first is the amyloidal form which includes cerebral amyloid angiopathy (CAA), a chronic degenerative disease. The second form is characterized as non-amyloidal form of cSVD which is often related to common vascular risk factors, such as elderly age, hypertension, diabetes mellitus, and many other factors [4].
Amyloidal cerebral small vessel disease
CAA is a common amyloidal form of cSVD. Incidences of CAA are mostly associated with advanced age [11]. It is caused by a progressive deposition of β-amyloid in the walls of cortical and leptomeningeal small arteries, which leads to vessel dysfunction and brain parenchymal injury. Deposition of β-amyloid is thought to be involved in vascular occlusion and rupture. CAA related vasculopathy includes features such as fibrinoid necrosis, loss of smooth muscle cells, wall thickening, microaneurysm formation, and perivascular blood breakdown with the resulting product deposition [12-16].
Histological diagnosis of CAA requires use of special staining for amyloid under light microscopy. CAA now is not only a cerebrovascular pathological disorder, but also a clinical syndrome and has a distinct phenotype of neuroimaging and neuropathology. Multiple lobar cerebral microbleeds (CMBs) or cortical superficial siderosis shows peculiar CAA manifestations on neuroimaging [11]. Recently, the use of positron emission tomography (PET) with amyloid tracers has been used to label vascular β-amyloid in patients with intracerebral hemorrhage (ICH) and might serve as a marker in future clinical trials [17].
Based on the specific location of amyloid deposition and allelic difference, at least two pathological subtypes of CAA have been identified: CAA type-1 characterized by amyloid in cortical capillaries, and CAA type-2, in which amyloid deposits are restricted to leptomeningeal and cortical arteries, but not capillaries [18]. Decreased cortical grey matter in the occipital lobe and decreased flux in the basilar artery were noted in patients of symptomatic hereditary cerebral hemorrhage with amyloidosis-Dutch type (HCHWA-D) [19]. The appearance of cortical thinning in patients with HCHWA-D indicated that vascular amyloid is an independent contributor to cortical atrophy. CAA-related cortical atrophy was facilitated by vascular dysfunction and even observed in the absence of Alzheimer’s disease [20]. Moreover, apolipoprotein E (APOE) gene polymorphism is associated with the two subtypes of CAA. APOE ε4 allele and APOE ε2 is legitimately associated with type-1 and type-2 diseases, respectively [15].
Non-amyloidal cerebral small vessel disease
In contrast to CAA, less specific and more difficult to define form of cSVD is broadly termed as non-amyloidal SVD. The term ‘‘hypertensive arteriopathy’’ is widely used to describe this form of cSVD which is often related to many vascular risk factors like hypertension, diabetes, etc. However, the term ‘‘hypertensive arteriopathy’’ is somewhat misleading in terms that it is not always necessarily related specifically to hypertension (accounted for other risk factors). This form of cSVD has also been enormously termed arteriolosclerosis, age-related or vascular risk factor related cSVD, or degenerative microangiopathy in various other reports [4,21].
‘‘Hypertensive arteriopathy’’ can be further divided on the grounds of structural histopathological abnormalities, e.g., distal atherosclerosis, arteriolosclerosis, lipohyalinosis (‘‘mural disease’’), fibrinoid necrosis, and microaneurysms. These subdivisions differ in distribution in micro vessels size and can coexist in various combinations. It is difficult to differentiate hypertensive and non-hypertensive pathological changes. Fibrinoid necrosis, and sometimes microaneurysm formation, is more common in hypertensive patients’ brains than in those without hypertension which eventually lead to deep PBH [10,22]. Nevertheless there is no consensus on the microscopic characterization of small vessel changes of “hypertensive arteriopathy” so that its severity is difficult to evaluate in any given case. Later epidemiological studies of patients with small deep infarcts has shown that the prevalence of hypertension, being more common, seemed no different than the patients with large artery extracranial and intracranial occlusive disease, which raised doubt about the importance of hypertension in causing the vascular changes that leads to these small deep infarcts [23].
Collagens are components of the extracellular matrix and are found in the normal vascular wall, where they usually are weakly immunolabelled. Over expression of fibrillar collagen type-III as well as strong immunoreactivity of the basement membrane component collagen type-IV in vascular smooth muscle cells were marked to be associated with non-amyloid microangiopathy [24]. It has been associated with white matter hyperintensities (WMHs) [25], enlarged perivascular spaces (PVSs) in basal ganglia [26], lacunar infarcts [27], and ICH in the several previous literatures. Comparative evaluation is carried out for detailed understanding of these two forms of cSVD (Table 1).
Genetic types of cerebral small vessel disease
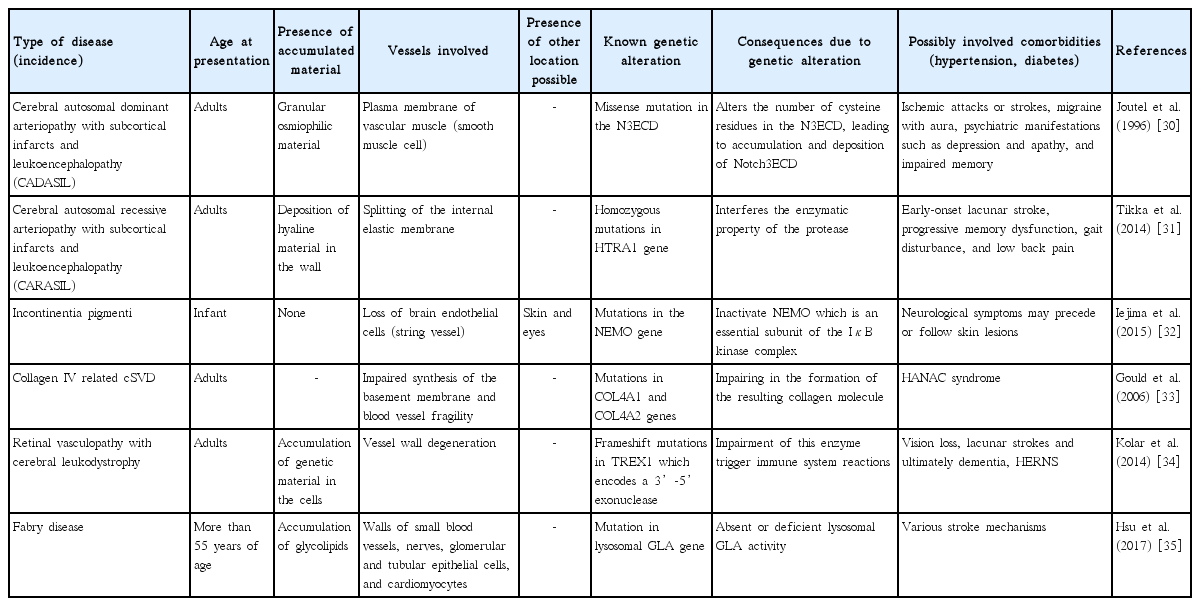
Genetics may play an important role in elucidating the cellular and molecular mechanisms and thus the pathophysiology of hereditary forms of cSVD. Genetic factors and their pathways have been elaborated for cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL), incontinentia pigmenti, and several other forms of cSVD. Sometimes, common denominators in monogenic SVD may contribute to disease progression. Even, distinct genetic mechanisms have been suggested in patients with lacunar stroke of different forms of cSVD [28]. Genetic studies are also important for elucidation of pathways involved and their interrelationship with other diseases involving brain’s white matter [29]. Cellular, molecular, and biochemical changes underlying cerebral small vessel damage can easily be assessed using animal models of these rare single-gene disorders. Unraveling the genetic aspect of cSVD could lead to an improved understanding of pathogenesis of cSVD, diagnosis and treatment. Table 2 is presenting the summary of various genetic determinant of the cSVD along with some of its uniform descriptors [30-35].
Alteration in blood-brain barrier and pathogenesis of cerebral small vessel disease
Traditional risk factors such as hypertension or diabetes mellitus play their important role in development of cSVD, but the exact pathogenesis of cSVD is still unclear [36]. Increased permeability of the BBB and endothelial dysfunction has been found to be associated with cSVD in several lines of evidence. BBB disruption is important pathological features of cSVD. Thus, circulating biologic markers of endothelial dysfunction might play a crucial role in identification of cSVD [37,38].
Blood-brain barrier
The BBB is a specialized physical and functional barrier that protects the brain from both invading pathogens and circulating immune cells that may enter and cause damage. It is comprised of endothelial cells (ECs) with tight junctions between them. Tight junctions act as a primary defense of the BBB and prevent cells and molecules from passively crossing into the brain [39]. Damage to BBB thus allows the entry of pathogens or immune cells and disturbs the function of the brain [40].
A widely accepted hypothesis is that with increasing age and the presence of chronic hypertension, there is a loss of the ability to self-regulate cerebral blood flow in response to variations in blood pressure, this, together with higher arterial stiffness, produces an increased speed and pulsatility of flow in the cerebral arterioles. These hemodynamic changes would lead to damage in the cerebral endothelium of the BBB and an alteration of its permeability through an increase of the shear stress [41].
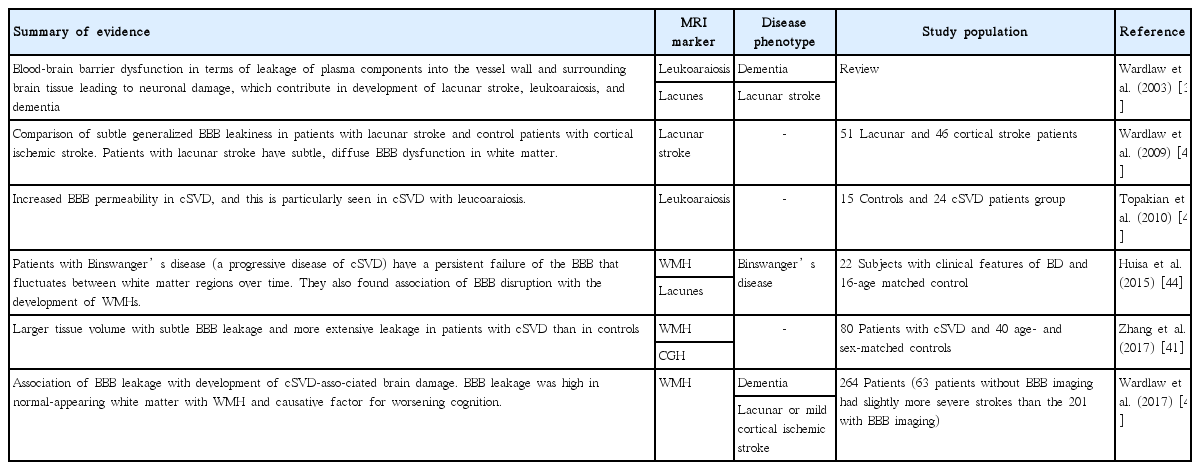
BBB leakage has been shown to be a common feature of cSVD supported by the accumulating lines of evidence (Table 3) [37,41-45]; therefore, endothelial dysfunction seems to be a pivotal factor contributing to the pathogenesis of cSVD [8]. Levels of circulating biomarkers of endothelial dysfunction have been found to be elevated in the blood corresponding to cSVD patients [38]. Although the role of other components of the BBB, including pericytes [46] and oligodendrocyte precursor cells (OPCs), has also been suggested in stabilization of BBB and these components are considered potential contributor to the microvascular damage in cSVD along with endothelium [47]. The fact of the matter is that various components of BBB interact with each other which play the pivotal role in the discovery and development of new therapies.
Endothelial dysfunction
Endothelial dysfunction has been found to lead to cSVD by various mechanisms. One of the hypotheses suggests the reduction in cerebral flow (hypoperfusion) in cSVD patients [48]. Cerebral flow is regulated by nitric oxide signaling which has been identified as a marker for endothelial dysfunction [49]. In another study, endothelial failure and BBB integrity was found to be associated with severity of WMH and there was significant decrease in integrity of ECs in WMH compared with normal white matter [50]. Another line of evidence suggests the increased BBB permeability due to endothelial dysfunction subsequently leads to brain parenchyma lesion. Support in this argument has been documented with higher cerebrospinal fluid (CSF)/serum albumin (SA) ratio (marker of BBB disruption) in dementia and patients with leukoaraiosis [37]. Figure 1 depicts the alteration in BBB and endothelial dysfunction in cSVD. Figure 2 shows the molecular mechanism of several hereditary forms of cSVD.

Alteration in blood-brain barrier (BBB) and endothelial dysfunction in cerebral small vessel disease. (A) Schematic representation of the BBB in normal condition (healthy individual), which consists of the monolayer of endothelial cell, connected by tight junctions and resting on the basal lamina. Circulating blood cells, such as neutrophils and monocytes, are also part of the unit, given the close interaction with the luminal surface of endothelial cells and their role in immune surveillance. Tight junctions consist of three main groups of proteins. They are transmembrane proteins (claudins, occludin, cadherins) and accessory proteins. These proteins interact to form a barrier from which minimal passive extravasation of plasma proteins, inorganic solutes or even water molecules occur. (B) Disassembly of proteins forming tight junction causes disruption of tight junctions leading to increased BBB permeability to small and large macromolecules. (C) Progressive BBB damage and leakiness results in stiffening of the vessel wall due to degradation of basement membrane (BM) and accumulation of extracellular matrix component. Leakiness in BBB also leads to immune cell infilteration and inflammation. TJ, tight junction; AJ, adherence junction; ZO, zonula occludens; MM-9, matrix metalloproteinases-9.

Molecular mechanism of several hereditary forms of cerebral small vessel disease namely cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL), and incontinentia pigmenti and their pathophysiology in small vessel causing a disturbed blood-brain barrier (image courtesy: AtheroPoint™, Atheropoint, Roseville, CA, USA). SMAD, mothers against decapentaplegic homolog; NEMO, nuclear factor κB (NF-κB) essential modulator; IKK, IκB kinase; TAK, TGF-β–activated kinase; TAB, TGF-β activated kinase 1 (MAP3K7) binding protein; TNFR, tumor necrosis factor receptor; GOM, granular osmophilic material; TGF-βR, transforming growth factor β receptor; ECM, extracellular matrix; LTBP-1, latent transforming growth factor β binding protein 1; LAP, latency-associated protein; HTRA1, high-temperature requirement a serine peptidase 1; N3ECD, extracellular domain of NOTCH3.
Biological markers of endothelial dysfunction
Evidence of endothelial failure in cSVD has begun to emerge in the form of elevated levels of markers for endothelial dysfunction in the blood of cSVD patients. In many of the studies, although circulating biomarkers were evaluated individually relying on single pathway, conceptually, different pathways should be taken in to the consideration to study several biomarkers simultaneously.
Inflammation markers: Higher expression of endothelial markers such as intracellular adhesion molecule 1, was proved to be associated with WMH progression, and supported the role of endothelial dysfunction in cSVD [51]. In many population-based cohorts, association between inflammatory markers and cSVD has also been reported. C-reactive protein is one abundantly studied marker in this line [52-54]. An independent association was reported with both lacunar infarcts and WMH in all the studies in which asymmetric dimethylarginine (ADMA) levels were measured [55,56]. Matrix metalloproteinase 9 has association with WMH [57]. Besides this, several other inflammatory endothelial biomarkers and their association with WMH and/or lacunar infarcts were also studied by many research groups. Some of these include E-selectine, neopterin, and vascular cell adhesion molecule [58]. Moreover, consistent association of ADMA [59] and circulating progenitor cell were suggested in CADASIL patients [60].
Hyperhomocysteinemia: In several population-based studies, increased level of homocysteine (HCY) and WMH were independently associated with silent lacunar infarcts [61-63].
Coagulation markers: Fibrinogen, a large plasma glycoprotein is synthesized in the liver [64]. Fibrinogen and its breakdown products are cleared from brain tissue by a local tissue plasminogen activator/plasminogen system [55]. Fibrinogen was assumed to be a faithful marker of BBB dysfunction for several reasons [65]. Interestingly, elevated levels of tissue factor pathway inhibitor, was found in lacunar infarct patients [66]. In a small study of Kario et al. [67] 2001, thrombin-antithrombin values were associated with the presence of WMH.
Serum albumin and albuminuria: During BBB dysfunction, albumin extravasations are prevalent in the aging brain and reflect BBB leakage. It is found to be associated with severe white matter lesions in cSVD [68]. Increased CSF/SA ratio, a marker of BBB breakdown, has also been reported in vascular dementia [69] and in patients with white matter changes on neuroimaging [70].
Albuminuria, an early marker of kidney disease [71] has also been proposed as a sensitive biomarker of systemic endothelial dysfunction [72]. There is documentation of an association between albuminuria and SVD neuroimaging markers. Many evidences suggest that peripheral systemic microvascular disease biomarkers could be useful in the evaluation of brain microvascular damage [73].
Serum neurofilament: Neurofilament (NfL) is an essential scaffolding protein of the neuronal cytoskeleton. On axonal damage, NfL is released into the extracellular space and subsequently into the CSF and blood and thus measuring NfL levels is a more direct approach to represent neuronal damage [5]. More recently, serum NfL is found to be increased in patients with a recent small subcortical infarct (RSSI). This has suggested NfL as a blood biomarker for active cSVD [74].
Cross talk among cellular components of BBB in cerebral small vessel disease
Abnormal endothelial functioning alone is not responsible in development of cSVD pathology. For the maintenance of BBB, other cellular components such as pericytes, astrocytes, and OPCs, are also thought to be essential although their exact contribution is yet to known. In fact, the impact of disrupted cross talk among BBB cell components in this regard is of great significant in understanding molecular mechanism and early phase identification of disease [75]. Interestingly, the role of pericytes in cSVD has been reported in CADASIL studies where genetic determinant of CADASIL (NOTCH3 gene), was shown to be expressed in pericyte that consequently contributed to pathogenesis by the abnormal interactions between pericytes and EC [48].
Changes to oligodendrocytes results in the loss of white matter integrity, which is an important aspect of cSVD. There are also many evidences that provide data of EC-oligodendrocyte signaling in cSVD. In general, endothelium secretes the factors responsible for survival and growth of OPC. Endothelial dysfunction in cSVD patients alters the secretion of releasing factors from ECs, which eventually affect oligodendrocyte survival and make them prone to damage [76]. One hypothesis suggests that signaling between dysfunctional ECs and oligodendrocytes may alter their ability to cope up to the damage caused by hypoperfusion in human with cSVD. Another hypothesis relies on the fact that angiogenesis in cSVD may impair migration of OPC upon blood vessels and thus reduce the repair process [47].
Cross talk of small and large vessel diseases
Cerebrovascular diseases and stroke are usually kept under large and small vessel domains and different types of pathologies thereof. However, their pathology affects each other in some or other way. It is supposed that large and small artery cross talk is responsible for causing cerebrovascular diseases [77] but whether large artery pathology alter small arteries or viceversa, is a question yet to be addressed. Even, spontaneously hypertensive rat (SHR) are being used both as a established model of small vessel arteriopathy and a large artery disease [78].
Many of recent studies established a fact that pathological mechanism of large vessel disease and SVD are distinct with each other [36]. Nevertheless, both processes may have their cooccurrence and could be overlapping. Interestingly, both subtypes of vascular disease share the similar risk factors such as aging and hypertension [79]. In many instances, increased large artery stiffness transmits the excessive flow pulsatility into the cerebral microcirculation as well as causing diastolic hypoperfusion which both damage microvascular wall thus leading to arteriolosclerosis and white matter damage [80-82]. Therefore, it would not be incorrect to say that both small and large artery disease make a continuum and interact dynamically. This hypothesis has been proved in several studies with animal models that have demonstrated disruption of the endothelial tight junction [83] and increase of white matter disease [84] in animal models with bilateral common carotid artery (CCA) stenosis. Moreover, arterial stiffness in carotid and femoral arteries has been associated with the presence of cSVD markers [85]. The same results along the line have shown decreased arterial elasticity of the CCA in cSVD patients when compared with normal individuals [86].
Imaging markers in cerebral small vessel disease
Neuroimaging plays a central role in the diagnosis and characterization of cSVD since parenchymal lesions caused by small vessel changes have been adopted as markers of cSVD [4]. Manifestation from neuroimaging has a wide spectrum ranging from RSSIs that can present with stroke to relatively more insidious clinically silent lesions. The silent lesions include WMH in the periventricular and deep white and grey matter, enlarged PVSs, lacunes, CMBs, and cerebral atrophy [87]. However, the definitions and terms of these lesions have varied significantly among studies. Therefore, an expert group in cSVD, Wardlaw et al. [88] proposed definitions and terminology for the structural neuroimaging features of cSVD to avoid confusion and improve clarity on cSVD in publication which is popularly referred as STandards for ReportIng Vascular changes on nEuroimaging (STRIVE). Neuroimaging features of SVD have been previously reviewed in depth [89]. Table 4 shows the details of each biomarker with their definitions and disease specific aspects. The cumulative effects of cSVD lesions can best determine the clinical impact and severity of the disease, rather than the individual lesions themselves [90].
cSVD can be visualized routinely on computed tomography and magnetic resonance imaging (MRI) of the brain. However, advanced imaging methods, including fluid-attenuated inversion recovery, T2-weighted, T1-weighted and gradient echo/T2*/susceptibility-weighted sequences and diffusion tensor imaging can provide a full spectrum of cSVD and thus will help in early detection of disease [91]. Figure 3 depicts the imaging of all four characteristic features of cSVD. Advances in imaging techniques have provided new insights into mechanisms of cSVD which consequently enables us to explore prevention and treatment strategy towards cSVD. Amyloid PET, widely being used in diagnosis of Alzheimer’s disease (AD), also has provided proof-of-concept data on CAA and its various manifestations [92]. Dynamic contrast-enhanced MRI has also been applied to measure permeability of BBB in cSVD patients [41]. It could also be used to elaborate permeability maps showing white matter permeability and could become an advanced diagnostic tool in cSVD diagnosis.

Neuroimaging markers of cerebral small vessel disease. (A) Recent small subcortical infarct on diffusion weighted imaging (arrow). (B) Lacune on fluid-attenuated inversion recovery imaging (FLAIR) (arrow). (C) White matter hyperintensity on FLAIR imaging (arrows). (D) Perivascular spaces on T1-weighted imaging (arrows). (E) Deep microbleeds on gradient recalled echo (GRE) T2 weighted imaging (arrows). (F) Lobar cerebral microbleeds on GRE imaging (arrows).
Total small vessel disease score
Recently, a ‘total SVD score’ term has been proposed which composite four established imaging biomarkers of cSVD (WMH, lacunes, PVS, and CMB) and estimate the overall burden of cSVD. A total SVD burden score is a better representation of the overall effect of cSVD on the brain rather than taking only one or two individual features separately on consideration. Total SVD burden is visually rated on a scale of 0 to 4. In this score, a point is allocated to each of the following: presence of lacunes, presence of CMB, moderate to severe PVS, and moderate to severe periventricular/deep WMH [90,93]. The total SVD score reflects overall burden of all markers and stratifies the severity of cSVD in a simple and pragmatic way. It may have potential of assessment of risk factors and interventions to prevent cSVD progression.
Machine/deep learning strategies for cerebral small vessel disease risk assessment
In medical imaging, image acquisition and image interpretation have both impacts upon accurate assessment of disease. Mostly, medical image interpretations require human observer, but interpretation by humans is limited due to large biasness and variations across interpreters, its subjectivity and human error [94]. Since medical imaging plays a prominent role in the diagnosis of cSVD, interests in application of computerized tools, specifically neural networking, machine learning, and deep learning in image processing have increased significantly for correct delineation of severity of cSVD.
Neural networks are a type of learning algorithm which comprises of neurons or units with some activation a and parameters; W, B, where W is a set of weights and B a set of biases [95]. Neural network is dominated in the field of health care because it has ability to capture the behavior of disease and to predict disease severity and its classification. Processes in neural networking comprised of medical image pre-processing, segmentation, object detection and recognition [96].
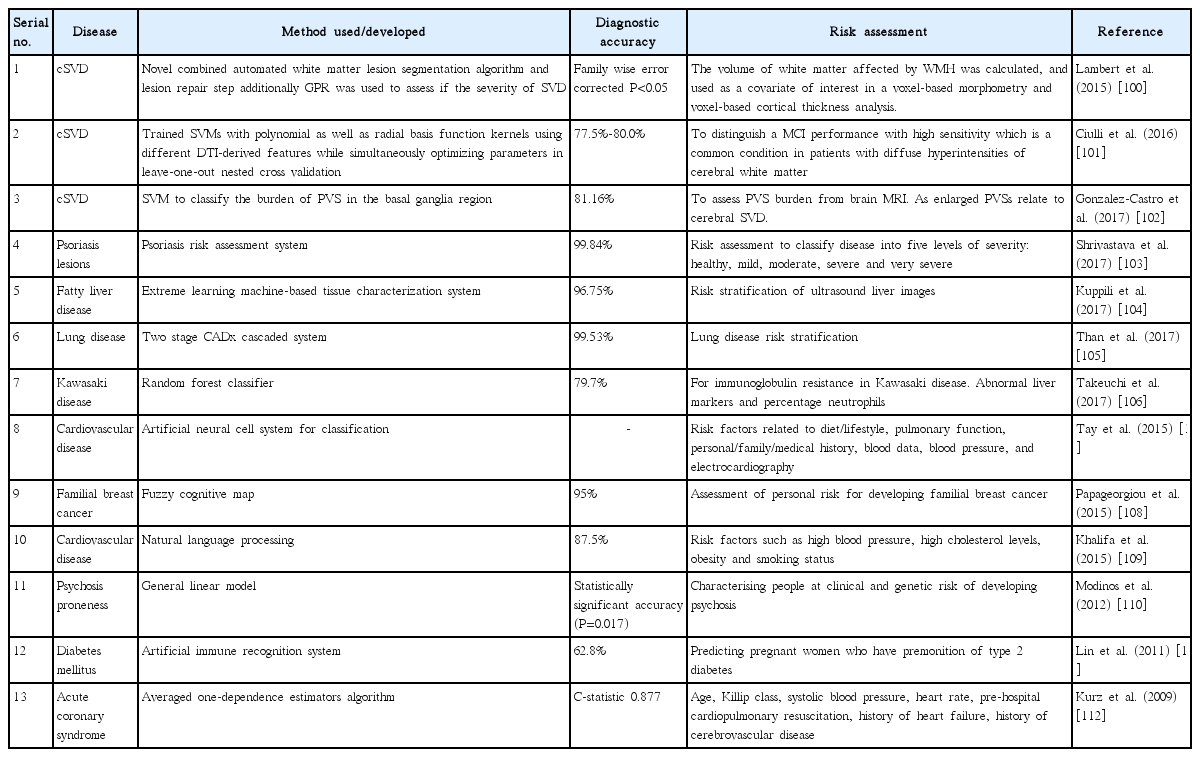
Machine learning is often recognized as a subdiscipline of neural networking. Concepts of machine learning have been applied to medical imaging for decades, most notably in the areas of computer-aided diagnosis, content-based image retrieval, automated assessment of image quality and functional brain mapping. Machine learning analyses are a data-driven approach, where algorithm works with a large number of possible predictor variables. In this supervised learning, the predictive model represents the assumed relationship between input variables in x and output variable y. The application of machine learning allows analytical flexibility in selecting the predictor variables, the settings of the machine learning analysis, and the clinical endpoint. To define endpoints apriori, pre-registration of analysis parameters could be carried out [97]. More recently, machine learning paradigm has been used for stroke risk stratification using ultrasonic echolucent carotid wall plaque morphology [98]. In another similar study, stroke risk stratification based on plaque tissue morphology using carotid ultrasound was improved in their performance by embedding pollingbased principal component analysis strategy into the machine learning framework to select and retain dominant features, resulting in superior performance [99]. Machine learning analyses compute and distinguish one feature from other by trained dataset. However, when several features are taken into consideration, it may give biased results. Table 5 dictating existing applications of machine learning in risk stratification of various diseases along with cSVD [100-112].
Deep learning is an improvement of artificial neural networks, and emerging as a promising machine learning tool in the medical imaging and domains of computer vision. It consists of more layers that permit higher levels of abstraction and improved predictions from data [113]. More specifically, Convolutional Neural Networks (CNN) have been recognized as a leading tool among groups of medical image analysis. CNN and other deep learning processes are giving outstanding results in applications of image analysis, interpretation, and risk stratification in current scenario. Nevertheless, CNN were applied as long ago as 1996 in medical image processing to the identification of biopsy-proven masses and normal tissues from mammograms [114]. Application of deep CNN in correct identification of cSVD markers and stratification in cSVD in to low, medium, and high severity is also well known undoubtedly. More recently, deep three-dimensional CNN were shown for automated detection of lacunes of presumed vascular origin. As the location information is important for the analysis of lacunes, network was equipped with contextual information using multiscale analysis and integration of explicit location features. Further, networks were trained, validated, and tested on a large dataset of 1,075 cases [115]. In another work, location sensitive deep CNN was proposed for segmentation of WMHs in MRI on large datasets. For this, network was integrated with the anatomical location information and shown to outperform a conventional segmentation method with both hand-crafted features and CNNs not integrated with location information [116].
The typical CNN architecture for image processing consists of a series of layers of convolution filters, interspersed with a series of data reduction or pooling layers. Figure 4 illustrates typical CNN architecture. The convolutional layers act as feature extractors, and thus they are learnt the feature representations of their input images. The neurons in the convolutional layers are arranged into feature maps. Filter bank (set of trainable weights) connects each neuron in a feature map to the neurons in the previous layer [113]. The convolution filters detect increasingly more relevant image features, and then higher order features. Pooling layers reduce the spatial resolution of the feature maps. Several convolutional and pooling layers are usually stacked on top of each other to form a deep model and retrieve more abstract feature representations. The fully connected layers interpret these feature representations and execute the function of high-level reasoning [94]. Deep learning is widely considered as current state-of-the-art leads to enhanced accuracy and took image analysis in cSVD to new horizon with respect to risk assessment, segmentation and stratification of progress of imaging markers and so cSVD.
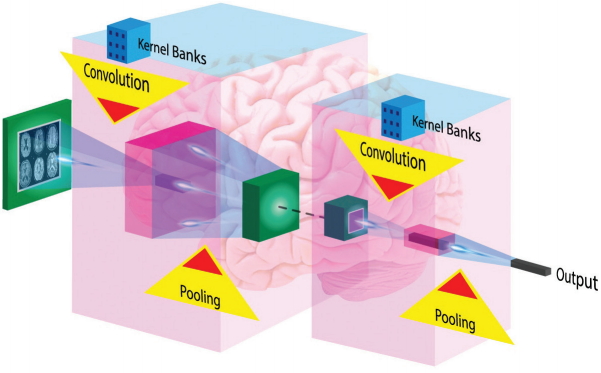
Architecture of typical Convolutional Neural Networks (CNN) for image processing. The typical CNN architecture for image processing consists of a series of layers of convolution filters, interspersed with a series of data reduction or pooling layers. Several convolutional and pooling layers are usually stacked on top of each other to form a deep model and retrieve more abstract feature representations. The fully connected layers interpret these feature representations and execute the function of high-level reasoning.
Cerebral small vessel disease associated diseases
This section presents special notes on associated diseases of cSVD with cerebral as well as non-cerebral component. Cerebral diseases include AD, Parkinson’s disease (PD) which is found to be closely related to cSVD. Non-cerebral diseases discussed here are subclinical hypothyroidism (SCH), hepatitis C virus (HCV) infection, and branch retinal artery occlusion (BRAO) which have direct or indirect association in pathology and occurrence of cSVD.
Relationship of cerebral small vessel disease and Alzheimer’s disease
There is much evidence suggesting the contribution of cSVD to the occurrence of AD. It has been well identified that there is an association of AD and key imaging markers of cSVD such as lacunar infarct, WMH, and CMBs [117]. Several studies showed the relationship between lacunar infarct, and components of Alzheimer’s pathology including amyloid β (Aβ) and tau pathology. Kandimalla et al. [118] implicates the association of higher Aβ42 and lower tau in AD. Lacunar infarctions could be tightly linked to higher levels of plasma Aβ40 and plasma Aβ40 in the AD patients [119]. Furthermore, CMBs in AD have been shown more closely coupled with CAA [120]. CMBs were also responsible for the alterations of Aβ metabolism in AD [121]. In another study, patients with early AD were detected by multiple CMBs in 7 T MRI tests [122]. It is also well established that WMH and cerebral atrophy are significant manifestations of AD [123] and also an imaging determinant of cSVD [124]. Another common clinical characteristic is cognitive decline and dementia, which is found in both AD and cSVD [125]. However, it remains unclear by what mechanism cSVD and AD pathology and their association impacts the cognitive decline and dementia. Evidence in this line came from the work of Kester et al. [126] who examined the deposition of amyloid in cSVD patients and revealed that levels were aggravated, especially APOE ε4 carriers, suggesting pathophysiological synergy between AD and cSVD. More recently, cSVD burden was found to be associated with selective disruption of cortical hub grey matter network integrity in AD brains [127]. It is suggested that treatment and prevention for cSVD can be beneficial for AD. Statins have been assumed to have role in treatment of neurodegeneration due to AD since it is beneficial in cSVD [117]. Cilostazol, a type-III phosphodiesterase inhibitor, has been shown to prevent white matter vacuolation and rarefaction induced by bilateral CCA occlusion in rats. Treatment of cilostazol has been demonstrated to improve BBB permeability and reduced gait disturbance, and visual impairment. When applied as an add-on therapy in AD patients, it is found to reduce the decline of cognitive function in those patients [128].
Relationship of cerebral small vessel disease and Parkinson’s disease
PD is the second most common neurodegenerative disease in the elderly. Recently, PD was evaluated as an important risk factor in the prevalence of ischemic stroke [129]. Additionally previous studies also suggested relationship between cSVD and mild parkinsonian sign (MPS), which are known as subtle motor disturbances. SVD may result in MPS by interfering with basal ganglia-thalamocortical circuits involving both the frontal and parietal lobes. Severe white matter lesions and the presence of lacunar infarcts were independently associated with the presence of MPS [130]. Therefore, cSVD imaging markers could be very useful in early detection of patients with motor impairment.
cSVD at baseline, has role in the etiology of parkinsonism. High WMH volume and a high number of lacunes was found to be directly associated with incident parkinsonism [131]. In this context, Hatate et al. [132] concluded that total SVD score might be helpful marker for MPS, especially deep CMB are of particular interest. In addition, cSVD cases had more frequent biomarker of neurologic diseases, such as brain stem raphe hypoechogenicity, substantia nigra hyperechogenicity and enlarged third ventricles. Substantia nigra hyperechogenicity was linked with gait disturbances, extrapyramidal features, and cognitive impairment. Brain stem raphe hypoechogenicity was correlated with the depression [133].
Relationship between cerebral small vessel disease and cognitive decline, dementia, and depression
Individual cSVD markers and total SVD score has been investigated in many cross-sectional [134,135] and longitudinal research studies [136]. The conclusion has been a significant association with lower/impaired cognitive function. New evidence in this line was reported recently in a longitudinal study which found significant cognitive decline particularly in executive function in concert with higher total cSVD score in patients with hypertension [5]. Earlier to this, an association of composite cSVD score was demonstrated with lower global cognitive function in particular impairment of executive function, language, and visuoconstruction [137]. Impact of cSVD on cognition depends on lesion location. Many case studies have suggested that small infarcts in the internal capsule, thalamus and caudate nuclei leads to marked cognitive impairment. Emerging evidence in the field is in the form of lesion-symptom mapping studies, which provided additional information on the impact of lesion location on the different domains of cognitive functioning [138].
Cognitive impairment in the elderly is often a mixed pathology (AD and cerebral microangiopathy) which may increase the risk of dementia [139]. CMB were reported to be an independent factor to heighten the risk of dementia [140]. Clasmatodendrosis along with WMHs and frontal white matter changes, is another contributing factor in cSVD dementia [141]. It is also observed that high levels of total HCY do well to predict the incident and risk factors of dementia. Thus, it could be an effective target in the prevention of dementia [142].
Depressive symptoms are also a major clinical manifestation of cSVD. However, they are largely poorly investigated [143]. WMH, lacunar infarcts, and CMB were associated with more severe depressive symptoms [144]. In another finding, loss in white matter integrity was directly related to depressive symptoms in cSVD patients [145].
Relationship of cerebral small vessel disease with epilepsy and gait disorder
The connection between cSVD and epilepsy has been under-researched. Some reports suggest that cSVD and hypertension may implicit the risk of epilepsy [146,147]. Experimental studies on SHRs (the model of cSVD) suggested predisposal of cSVD to temporal lobe epilepsy more than any other type of epilepsy [148].
Gait and balance impairment highly prevails in the elderly, which increases the risk of falls, institutionalization, and mortality. Gait disorder is considered the second most common consequence of cSVD after cognitive impairment [149]. Gait disturbances followed by incontinence and dementia were considered as clinical symptoms of normal pressure hydrocephalus (NPH), a disorder found mainly in the elderly with age above 60 years. More interestingly, PD, AD, and vascular dementia have high comorbidity with NPH [150]. In a recent study, the most frequent initial symptoms of NPH were determined which was gait impairment and cognitive decline in men and women, respectively, and the most frequent comorbidities were hypertension and diabetes in men and women, respectively [151].
Most investigations relied only on a single imaging marker of the cSVD and its association with gait and balance disturbance [152,153]. More recently, the impact of individual cSVD markers and global SVD score together has been investigated in gait speed. Among cSVD markers WMH was the significant driving force on a patient’s performance in the gait impairment.
A short note on cerebral small vessel disease and associated non-cerebral diseases
Some clinical disorders, which do not have any direct connection with brain, have been found to be associated with vascular resistance, arterial wall stiffening and endothelial dysfunction, which in turn render them for possessing a potential relationship with cSVD.
SCH has been related with atherosclerosis and elevated risk of ischemic stroke. Zhang et al. [154] elucidated the association of SCH with the presence of WMH, CMB, and composite cSVD burden in patients with transient ischemic stroke. In another report, correlation of arteriolar wall thickness was studied with many factors including HCV and human immunodeficiency virus (HIV) infection and concluded that HCV, not HIV establishes an independent risk factor to cerebrovascular pathology [155]. However, some previous studies suggested the increased risk of ischemic stroke in HIV infected patients [156,157]. Patients receiving high active antiretroviral therapy (HAART), in particular, suffered with injury to their vessel walls or metabolic abnormalities that eventually provoked the development of atherosclerotic large vessel disease and myocardial infarction [158]. Furthermore, the potential impact of HAART on small vessel results in cognitive impairment and abnormal metabolism in HIV-infected adults [159]. Another earlier finding suggested that WMHs are associated with HIV seropositive individuals [160]. Recently, during investigation of the BRAO, it was suggested that small vessel etiology may play a pivotal role in pathophysiology of BRAO [161]. The above investigations give another dimension of cSVD research and exploration in the direction of linkage to several other non-cerebral disorders.
Conclusions
Advancement in the elucidation of the disease mechanism will allow us to increase the understanding of the disease pathways, pathophysiology, and common denominator of cSVD which will be the key for innovation in molecular target therapy. BBB rescuing could shed light on other treatment options. MRI markers for cSVD and assessment of global burden by total SVD score provide risk stratification for cSVD in clinical settings and play a crucial role in clinical research. Advancement in image classification processes by embellishing it with stateof-the-art tools of deep learning and big data has enabled correct delineation of the disease and accurate assessment of severity of cSVD.
Notes
Disclosure
The authors have no financial conflicts of interest.