Prognosis of Acute Intracranial Atherosclerosis-Related Occlusion after Endovascular Treatment
Article information
Abstract
Background and Purpose
Little is known about prognosis after endovascular therapy (EVT) for acute large artery occlusion (LAO) caused by underlying intracranial atherosclerotic stenosis (ICAS). Therefore, we investigated the prognosis following EVT according to the underlying etiology of LAO.
Methods
Patients from the Acute Stroke due to Intracranial Atherosclerotic occlusion and Neurointervention-Korean Retrospective (ASIAN KR) registry (n=720) were included if their occlusion was in the intracranial anterior circulation and their onset-to-puncture time was <24 hours. Occlusion was classified according to etiology as follows: no significant stenosis after recanalization (Embolic group), and fixed significant focal stenosis in the occlusion site with flow impairment or re-occlusion observed during EVT (ICAS group). Patients were excluded when significant extracranial carotid lesions existed, and when the intracranial occlusion was intractable to EVT so that the etiology was undetermined. The effect of angiographic etiologic classification on outcomes was evaluated using multivariable analysis that was adjusted for potential confounders.
Results
Among eligible patients (n=520), 421 and 99 were classified in the Embolic and ICAS groups, respectively. Patients in the Embolic and ICAS groups had similar successful reperfusion rates with EVT (79.6% vs. 76.8%, P=0.537) and 3-month functional independence (54.5% vs. 45.5%, P=0.104). In multivariable analysis, ICAS-related occlusion (odds ratio, 0.495; 95% confidence interval, 0.269 to 0.913; P=0.024) showed poorer 3-month functional independence compared to embolic occlusion.
Conclusions
After EVT, patients with acute ICAS-related occlusion have relatively poor functional outcomes compared to those with embolic occlusion. Novel strategies need to be developed to improve EVT outcomes for ICAS occlusion.
Introduction
Several clinical trials have shown that endovascular treatment (EVT) using newer thrombectomy devices within an onset-to-puncture time of 6 to 12 hours has efficacy and safety superior to those of intravenous thrombolysis [1-5]. Trials conducted more recently have revealed that EVT has substantial efficacy even within an onset-to-randomization time of 16 to 24 hours in selected patients [6,7]. Although clinical practice guidelines have now been updated to reflect these advancements [8-11], the outcomes of EVT for acute large artery occlusion (LAO) presumably due to intracranial atherosclerotic stenosis (ICAS), which is encountered more frequently in Asian patients, have not yet been adequately addressed.
In acute occlusion of the posterior circulation, the prognosis of ICAS-related occlusion is reportedly worse than that of embolic occlusion [12]. In the anterior circulation, results of single-center anecdotal studies have shown comparable prognosis in ICAS-related occlusion [13,14]. However, the ICAS population in these studies was younger with lower initial severity; therefore, large multicenter studies that include patients with ICAS-related occlusion from endemic regions and those that control for confounding factors are warranted.
Therefore, in the present study, we retrospectively analyzed the prognosis of EVT for ICAS-related versus embolic occlusion of the anterior circulation by using prospectively collected data from a registry of information on patients at three comprehensive stroke centers in Korea.
Methods
Patient enrollment
The Acute Stroke due to Intracranial Atherosclerotic occlusion and Neurointervention-Korean Retrospective (ASIAN KR) registry was assembled for an observational study of consecutive patients aged ≥18 years who received EVT for the treatment of acute ischemic stroke due to intracranial and/or extracranial large vessel occlusion. Patient data were collected from three comprehensive stroke centers in Korea between January 2011 and February 2016 [15]. Some patients in the registry might have been included in previous pilot studies performed by each hospital [13,14]. All clinical data were de-identified and allocated study identification numbers. The data collection protocol was approved by the Institutional Review Board of each respective hospital and was implemented in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments.
Etiologic classification of target occlusive lesions
The etiology of target large vessel occlusion (i.e., embolic or ICAS-related occlusion) was determined by core laboratory imaging analyses based on angiographic diagnosis according to previous reports (Y.H.H. & J.S.L.) [12,14,16]. In brief, after confirmation of arterial occlusion, uncommon cerebral arterial diseases such as dissection, moyamoya disease, and vasculitis were evaluated. If the occluded vessel was completely recanalized after primary thrombectomy, the etiology was classified as embolic occlusion (Embolic group). A remnant stenosis of >70%, or a lesser degree of stenosis with a tendency toward reocclusion and/or flow impairment during the procedure, was classified as ICAS-related occlusion (ICAS group). If grading was difficult to determine or discordant, consensus was reached by the two graders (Y.H.H and J.S.L). In addition, this mechanism was further evaluated and could be amended by repeat angiography following EVT during admission (J.S.Y.). Consequently, Embolic and ICAS groups were included in the analyses.
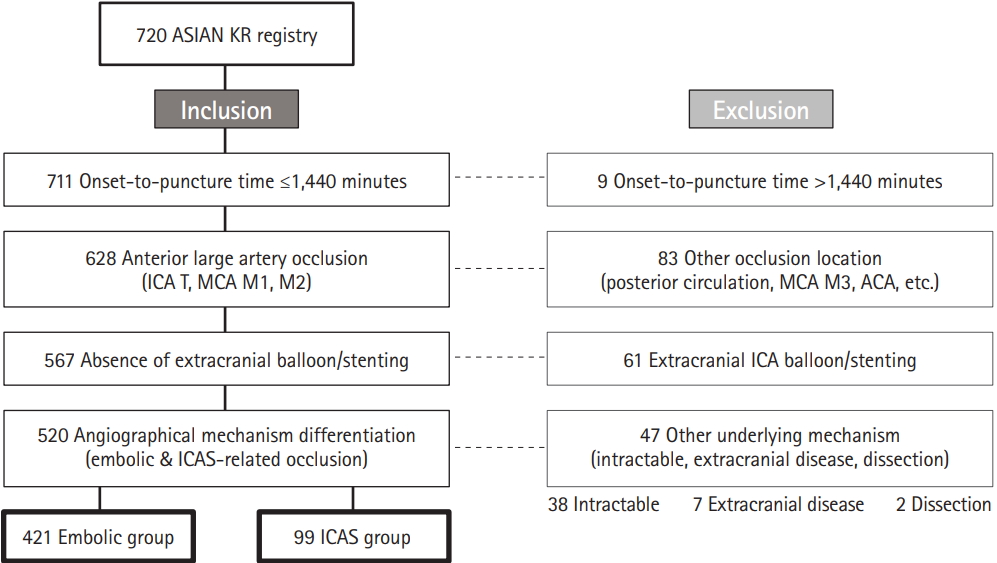
Inclusion and exclusion criteria
For the current study, the following inclusion criteria were applied: (1) acute intracranial LAO of the anterior circulation and (2) onset-to-puncture time of <1,440 minutes. The onset time was defined as last normal seen. Internal carotid artery (ICA) T, middle cerebral artery (MCA) M1, and MCA M2 occlusions were included in this study. Patients were excluded if (1) extracranial balloon angioplasty or stenting was performed; (2) angiographic etiology was classified as dissection, extracranial ICA stenosis-related, or undetermined; or (3) the occlusion was intractable to mechanical thrombectomy so that the etiology could not be differentiated.
Evaluations
Premorbid and 3-month modified Rankin Scale (mRS) scores and National Institutes of Health Stroke Scale (NIHSS) scores at admission were analyzed. A good outcome was defined as a 3-month mRS score of 0 to 2 or no change compared to the premorbid mRS. Results of routine laboratory tests were also collected. After de-identification and blinding of clinical data, stroke neurologists, neuroradiologists, and neurointerventionists with expertise in acute stroke management performed core laboratory imaging analyses to ensure consistent grading and to eliminate possible bias. The locations of initial large vessel occlusions were identified on baseline computed tomography (CT) or magnetic resonance angiography (S.J.L.). Alberta Stroke Program Early CT Scores (ASPECTS) were determined using noncontrast CT (S.I.S.). Final reperfusion was evaluated according to the modified treatment in cerebral ischemia (mTICI) grade [17]. Successful reperfusion was defined as mTICI grades 2b–3 (J.S.L.). Final recanalization was evaluated with arterial occlusive lesion grade [17]. Post-procedural intracerebral hemorrhages were classified in accordance with the criteria defined by the European Cooperative Acute Stroke Study (S.I.S.) [18]. Subarachnoid hemorrhage (SAH) was classified using the modified Fisher scale (S.I.S.) [19]. Serious hemorrhagic complications were defined as parenchymal hematoma type 2 and/or SAH grades 3 to 4 (thick SAH). Reocclusion was evaluated using repeat angiography performed during admission by comparing it with the last angiography performed during EVT (J.S.Y.).
Endovascular procedures
The type of EVT procedure was selected at the discretion of the treating physician. The direct aspiration method and stent retrieval were routinely used. The direct aspiration method refers to a forced arterial suction thrombectomy that uses the Penumbra system (Penumbra Inc., Alameda, CA, USA) [20,21]. Stent retrieval refers to clot removal by capturing and removing the thrombus with a stent retriever such as the Solitaire AB/FR (Medtronic, Irvine, CA, USA) or Trevo (Stryker, Kalamazoo, MI, USA) [22-24]. Balloon guide catheters, adjuvant lytic infusion, intracranial or extracranial angioplasty, and/or stenting were implemented as needed.
Statistical analysis
Comparative analyses between the Embolic and ICAS groups were performed for clinical characteristics, imaging findings, and treatment outcomes. Differences among the groups were analyzed using chi-square tests for categorical variables, Mann-Whitney tests for ordinal variables, or t-tests for continuous variables. To evaluate the effect of underlying etiology on good outcomes, we performed a multivariable analysis that was adjusted for the following potential confounders: age, sex, premorbid mRS, initial NIHSS score, pretreatment ASPECTS, intravenous tissue plasminogen activator use, onset-to-puncture time (minute), procedure time (minute), final successful reperfusion, and serious hemorrhagic complications. An interaction between underlying etiology and some influential variables on patient outcomes was also evaluated with adjustment of the same confounding variables except procedure time. Regression lines of embolic occlusion and ICAS-related occlusion were drawn for the probability of good outcomes versus onset-topuncture time, age, NIHSS score, and ASPECTS (unadjusted). A P<0.05 was considered significant. Statistical analysis was performed using the SPSS statistical package version 22.0 (IBM Co., Armonk, NY, USA).
Results
Classification process and final grouping
Among the 720 patients enrolled in the registry, 520 were included in the current study (Figure 1). We performed a blinded evaluation of the underlying etiology, using EVT angiographies combined with the evaluation of repeat angiographies during admission following EVT (608 of 720 patients). After exclusion criteria were applied regarding onset-to-puncture time, occlusion location, and extracranial ICA treatment, 567 patients were included. Among these, the classification was changed into other category from etiology based on EVT angiography in 19 patients after evaluation of repeat angiography. Among the 19 patients, two were changed from ICAS etiology to embolism while 11 were changed from embolism to ICAS. Among intractable cases, one patient had proven ICAS while five had embolism. Finally, the Embolic and ICAS-related groups comprised 421 and 99 patients, respectively.
Differences in baseline characteristics
Table 1 describes the baseline clinical and laboratory data of the groups. Compared with the Embolic group patients, the ICAS group patients were significantly younger and mostly men, with a higher prevalence of a history of smoking. In the ICAS group, occlusive lesions were relatively more frequent in the MCA M1 portion, and these patients presented with significantly lower initial NIHSS scores than those in the Embolic group. In terms of laboratory data, the patients with ICAS presented with significantly higher levels of total cholesterol, triglycerides, and low density lipoprotein cholesterol than those in the Embolic group, while levels of glucose, glycosylated hemoglobin, and inflammation markers did not differ between groups.
Differences in treatment and outcomes
Table 2 shows the comparison of treatments and outcomes according to etiological classification. Compared to the Embolic group, the ICAS group had a significantly longer median onsetto-puncture time (median, 235 and 320 minutes, P=0.002), and median total procedure time (median, 55 and 68 minutes, P=0.001), reflecting the complexity of the procedure. Concerning the final endovascular outcomes, the rates of successful reperfusion were similar in the Embolic and ICAS groups (79.6% vs. 76.0%, P=0.484).
No significant difference was observed between the groups in the grades and frequency of post-procedural intracerebral hemorrhage and the rate of serious hemorrhagic complications. On repeat angiography, reocclusion occurred in nine of 362 patients (2.5%) in the Embolic group and 14 of 89 patients (15.7%) in the ICAS group, based on the last angiography performed during EVT (P<0.001).
Good outcomes at 3 months were achieved in 54.5% and 45.5% of patients in the Embolic and ICAS groups, respectively (P=0.104), and the ordinal distribution of mRS scores was not significantly different between these groups (P=0.629) (Figure 2). However, when angiographic etiology was included along with potential confounders in a logistic regression model to predict good outcomes at 3 months, the prognosis of ICAS-related occlusion was worse than that of embolic occlusion (odds ratio, 0.495; 95% confidence interval, 0.269 to 0.913; P=0.024) (Table 3). An interaction of underlying etiology on patient outcomes was observed with procedure time per 30 minutes (P=0.046), but not with the number of EVT techniques (P=0.214).
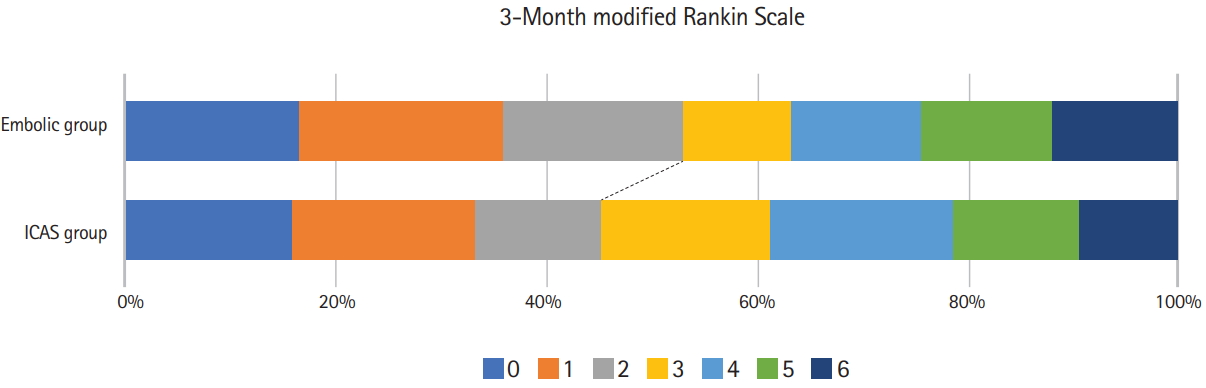
Modified Rankin Scale (mRS) scores at 3 months. Without adjustments, the distribution of mRS scores and the frequency of good outcomes did not differ between the Embolic and intracranial atherosclerotic stenosis (ICAS) groups.
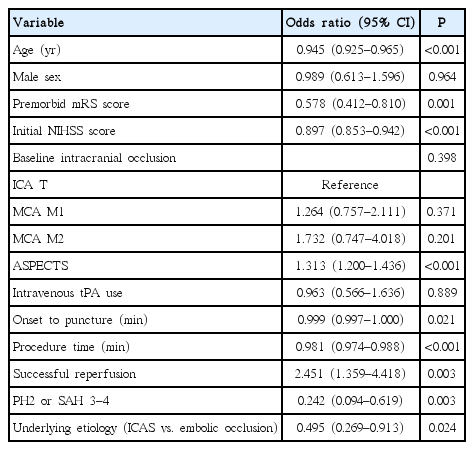
Results of logistic regression model for evaluating the association between occlusion etiology and good outcome
Graphs of onset-to-puncture time versus the probability of good outcomes at 3 months revealed that the pattern of regression lines varied between embolic and ICAS-related occlusion (Figure 3A). While the probability of good outcomes in patients with embolic occlusion declined as onset-to-puncture time increased, the probability of good outcomes in patients with ICAS-related occlusion did not decline but tended to increase with an increase in onset-to-puncture time. As for age, NIHSS score, and ASPECTS, the probability tended to be lower in the ICAS group than in the Embolic group, although the differences were not statistically significant (Figure 3B-D).
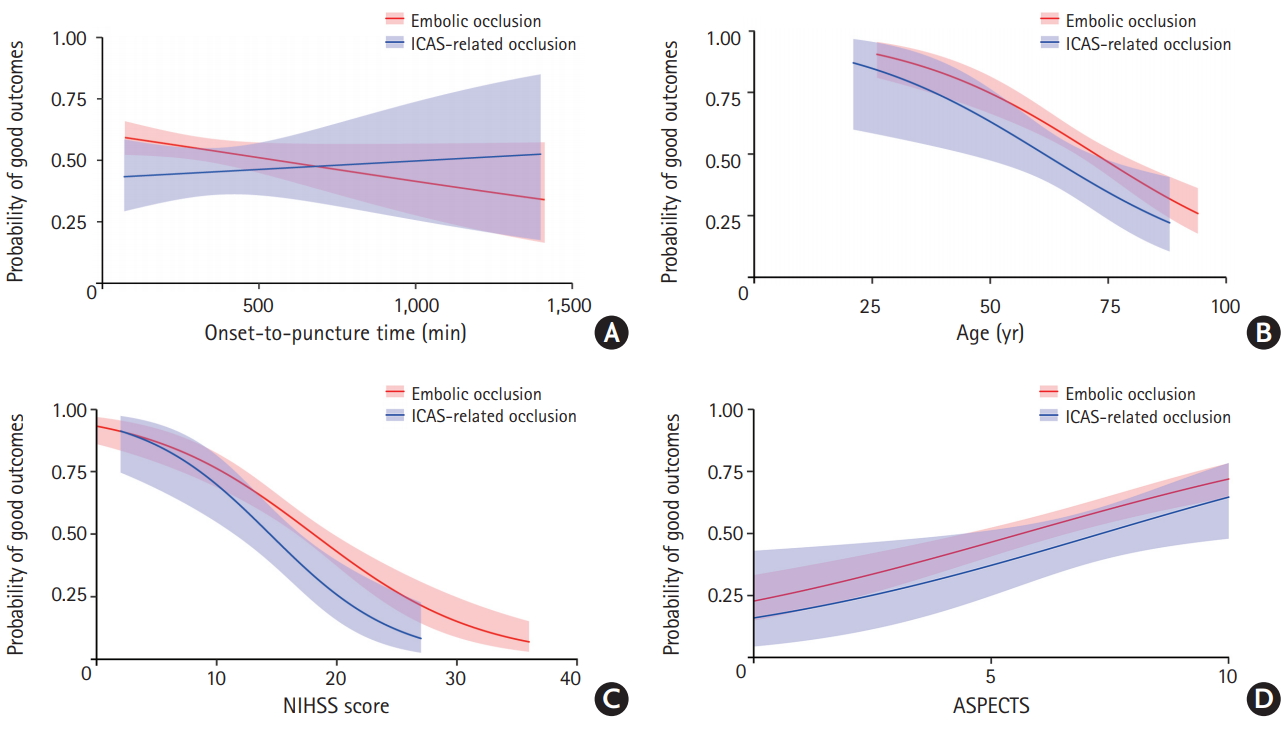
Relationship between good outcomes and well-known predictors in endovascular treatment for acute ischemic stroke and comparison between embolic and intracranial atherosclerotic stenosis (ICAS)-related occlusion. (A) The probability of good 3-month outcomes tended to decline in the Embolic group but stayed stable in the ICAS group as onset-to-puncture time increased up to 24 hours. (B-D) The probability of good outcomes appeared to be somewhat less in ICAS-related occlusion than in embolic occlusion; however, this was not statistically significant. NIHSS, National Institutes of Health Stroke Scale; ASPECTS, Alberta Stroke Program Early CT Scores.
Supplementary Document, in which patients with atrial fibrillation were excluded from the ICAS group, are shown in the online site. The results from patients with ICAS-related occlusion but without atrial fibrillation are similar to the above results obtained from patients with ICAS occlusion with or without atrial fibrillation.
Discussion
The major finding of the current study was that the prognosis of EVT for ICAS-related occlusion in the anterior circulation was relatively worse than that of EVT for embolic occlusion, indicating that better therapeutic approaches are required for patients with ICAS-related occlusion. Compared to patients with embolic occlusion, those with ICAS-related occlusion had lower initial NIHSS score and milder baseline ASPECTS, and were younger; these factors are known to be associated with good outcomes after EVT. Although the absolute rates of successful reperfusion were similar between the ICAS and Embolic groups, the outcomes of ICAS-related occlusion were worse than expected after adjustment for major confounders.
The relatively poor outcome in the ICAS group is mainly attributable to longer procedure time, reflecting the procedure complexity, and the higher rate of reocclusion. From our analysis, an interaction of underlying etiology and procedure time (per 30 minutes) was observed with patient outcomes. A meta-analysis from recent endovascular trials showed that time interval is more important in the hospital (door to reperfusion or image to reperfusion) than before hospital arrival (symptom onset to hospital arrival) [25]. Procedure time is a major portion of the in-hospital time; thus, the longer procedure time in the ICAS group could have contributed to the worse outcomes. Another factor is reocclusion tendency in ICAS-related occlusion. Reocclusion after EVT can lead to early neurological deterioration [14,26]. Reocclusion was more frequently seen in the ICAS group than in the Embolic group. Moreover, during EVT, ICAS-related occlusion tends to have a high rate of refractoriness to mechanical thrombectomy due to repeat reocclusion, which necessitates additional rescue treatments such as intracranial stenting or lytic treatments, and eventually delays final reperfusion time [27-31].
Nevertheless, considering the relatively high rate of good outcomes (45.3%) in the ICAS group, which is similar to the 46% (33% to 71%) in pooled data from five early-window trials (HERMES) and 47% (45% to 49%) in two late-window trials [32,33], EVT using contemporary devices and methods can achieve substantial performance and satisfactory outcomes even for ICAS-related occlusions. This is particularly relevant to clinical practice in Asian countries, because Asian patients, who have a higher incidence of ICAS-related occlusions [16], are significantly underrepresented in successful EVT trials [1-7].
The probability of good outcomes did not decline in these patients with an onset-to-puncture time of up to 24 hours, whereas it declined in patients with embolic occlusion and an increase in onset-to-puncture time. ICAS-related occlusion may have more opportunities for EVT because the ICAS prognosis patterns are similar to those associated with the “late window paradox” observed in recent trials conducted to demonstrate the efficacy of EVT in the late window (up to 24 hours onset-to-randomization time) [6,7,33]. Previous studies have indicated that good outcomes were unlikely in patients with evidently poor collaterals [34,35]; patients with both rapidly and slowly failing collaterals can be ideal candidates for EVT within the usual early window (less than 60 to 300 minutes of onsetto-puncture time) [35,36]. In patients with universally good collaterals, tiny-to-small infarcts are expected; therefore, these patients were not considered good candidates for EVT [35]. However, recent late-window trials have shown an absolute increase of 32% in good outcome rates compared to 19% in early window trials [32,33]. Patients with preformed ICAS can have universally good collaterals, which are developed by hypoperfusion with stenosis [37]. In an anecdotal study, the baseline infarct core volume was significantly lower in patients with ICAS-related occlusion compared to those with embolic occlusion [38]. Compared to early window trials using target artery occlusion and small core volume mostly based on noncontrast CT such as ASPECTS, these late window trials are based on infarct core volume with clinical or perfusion mismatch. Further imaging studies are warranted to incorporate this concept of “late window paradox” in the treatment of ICAS-related occlusion.
Although imaging predictors should be studied further, differences in baseline characteristics including demographics and risk factors may differentiate ICAS-related occlusion from embolic occlusion. Besides younger age and male predominance, MCA M1 occlusion was more frequent among locations of occlusive lesions in patients with ICAS. Well-known risk factors for atherosclerosis were also documented in our patients with ICAS, as current smokers were more frequent, and levels of total and low-density cholesterol and triglycerides were higher. Our study members plan to further investigate differential factors of various imaging tools. In addition, better treatment methods for ICAS-related occlusion will be further evaluated using the ASIAN KR registry.
The study has several limitations. First, this was a retrospective study with data from three Korean centers and included patients treated with somewhat outdated devices such as the first-generation Penumbra system. Thus, the results of this study are not generalizable to the entire Korean or Asian population or to contemporary treatments. Second, based on the analyses of a current real-world study, patients whose occlusion failed to open were excluded. If the intractable cases accounted for more patients with embolic occlusion, i.e., those with greater age and higher initial NIHSS scores, the poor outcomes in the Embolic group might be underestimated. However, those with ICAS-related occlusion had longer onset-topuncture and procedure times and these important confounding factors had been adjusted by logistic regression analysis, so it is believed that the current results are not significantly deviated. Lastly, although etiological classification was based on an evaluation of angiography during EVT and was confirmed by further analysis with repeat angiography during admission, this assessment might still be incomplete. In patients with ICAS-related occlusion, the frequency of atrial fibrillation was 24%. However, the baseline characteristics and risk factors in the ICAS group were significantly different from those in the Embolic group, and the analytic results were not significantly changed when atrial fibrillation was excluded from the ICAS group (shown in the Supplementary Tables 1-3); thus, our main outcome, i.e., prognosis, is believed to be accurately reflected in our results.
In conclusion, our study showed that following EVT, patients with acute ischemic stroke due to ICAS-related occlusion had a relatively poorer prognosis than patients with acute stroke due to embolic occlusion.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2018.01627.
Comparison of baseline characteristics and risk factors among groups
Comparison of treatment and outcomes among groups
Results of logistic regression model for evaluating the association between occlusion etiologies and good outcomes
Notes
Disclosure
The authors have no financial conflicts of interest.
Acknowledgements
This work was partly supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea Government (MSIP) (No. 2014R1A5A2010008: Sung-Il Sohn; NRF-2018R1A2B6007094: Jin Soo Lee).