Non-Vitamin K Antagonist Oral Anticoagulants in Medical Conditions at High Risk of Thromboembolism beyond Atrial Fibrillation
Article information
Abstract
Non-Vitamin K antagonist oral anticoagulants (NOACs) have been extensively investigated in medical conditions at high risk of venous or arterial thrombosis other than atrial fibrillation (AF), including hip or knee arthroplasty, acute venous thromboembolism (VTE), cancer-associated VTE, acute coronary syndrome (ACS), stable atherosclerotic vascular disease, chronic heart failure, and embolic stroke of undetermined source (ESUS). Two large ESUS trials failed to show the benefit of rivaroxaban or dabigatran, and large randomized controlled trial (RCT) data of NOACs are lacking for another potential candidates of patent foramen ovale-related stroke, acute ischemic stroke, and cerebral venous thrombosis. On the other hand, high quality evidences of NOACs have been compiled for VTE prophylaxis after hip or knee arthroplasty, acute VTE, cancer-associated VTE, and concomitant ACS and AF, which have been reflected in clinical practice guidelines. In addition, RCTs showed the benefit of very low dose rivaroxaban in combination with antiplatelet therapy in patients with ACS and in those with stable cardiovascular disease. This article summarizes the accumulated evidences of NOACs in cardiovascular diseases beyond AF, and aims to inform healthcare providers of optimal regimens tailored to individual medical conditions and help investigators design future clinical trials.
Introduction
Pivotal randomized controlled trials (RCTs) demonstrated the efficacy and safety of non-vitamin K antagonist oral anticoagulants (NOACs) for stroke prevention in atrial fibrillation (AF) [1-4], and major AF guidelines recommend NOACs over warfarin [5,6]. Even before the first RCT in AF was published in 2009 [1], large RCTs had already demonstrated the benefit of NOACs for preventing venous thromboembolism (VTE) in patients undergoing hip or knee arthroplasty [7-11]. These RCT findings and the convenience for clinical use accelerated the investigation of NOACs in diverse medical conditions at high risk of venous or arterial thrombosis. In particular, large RCTs have been completed or are ongoing in acute VTE with or without cancer, acute coronary syndrome (ACS) with or without AF, stable atherosclerotic vascular disease, chronic heart failure (CHF), and embolic stroke of undetermined source (ESUS), and the evidences from pivotal RCTs have been reflected in clinical practice guidelines. The NOAC regimens with proven efficacy in individual medical conditions are variable with regard to dose, duration, and combination with other antithrombotics, and thereby physicians should recognize optimal regimens for specific conditions to maximize benefit and minimize risk.
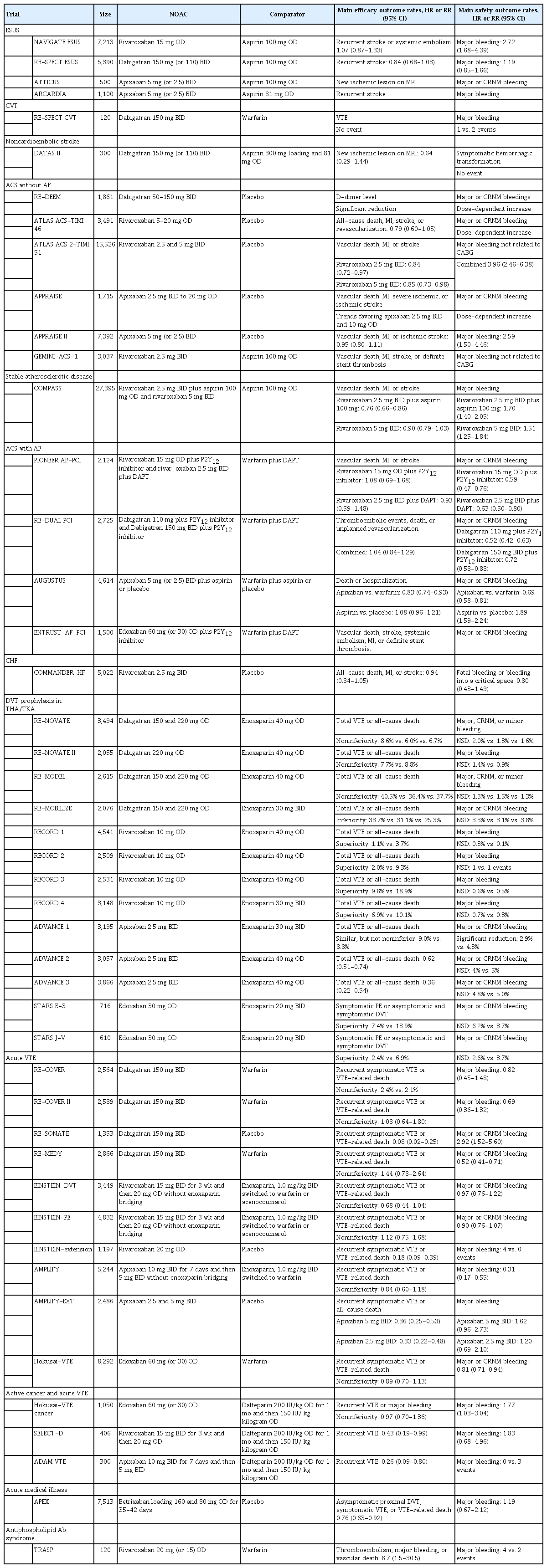
This topical review summarizes the trial findings of NOACs in diverse medical conditions other than AF (Table 1), and aims to inform healthcare providers of the accumulated evidences applicable to clinical practice. Investigators should also acknowledge the lessons from the earlier successful and unsuccessful trials to design future clinical trials.
Embolic stroke of undetermined source
ESUS, nonlacunar ischemic strokes without well-recognized cardioembolic source or >50% proximal arterial stenosis [12], accounts for approximately 20% of ischemic strokes, and has a high risk of recurrent stroke even with antiplatelet therapy. The proposed stroke mechanisms in ESUS include cardiac embolism associated with minor-risk cardioembolic sources or undetected AF, arterial embolism from ulcerative plaques of <50% proximal arterial stenosis or aorta, paradoxical embolism, or hypercoagulable status associated with covert cancer or systemic diseases. Based on the favorable benefit-risk profiles observed in pivotal AF trials, it was hypothesized that NOACs compared to antiplatelet therapy would be more effective to prevent recurrent stroke in patients with ESUS. To date, New Approach Rivaroxaban Inhibition of Factor Xa in a Global Trial versus ASA to Prevent Embolism in Embolic Stroke of Undetermined Source (NAVIGATE ESUS) and Randomized, double-blind, Evaluation in secondary Stroke Prevention comparing the EfficaCy and safety of the oral Thrombin inhibitor dabigatran etexilate vs. acetylsalicylic acid in patients with Embolic Stroke of Undetermined Source (RE-SPECT ESUS) have been completed [13,14], and AtRial Cardiopathy and Antithrombotic Drugs In prevention After cryptogenic stroke (ARCARDIA) and Apixaban for Treatment of Embolic Stroke of Undetermined Source (ATTICUS) are underway [15,16].
NAVIGATE ESUS (n=7,213) compared rivaroxaban (15 mg once daily [OD]) and aspirin (100 mg OD). The median interval from stroke onset to randomization was 37 days. The trial was early terminated because of a higher bleeding risk and little chance of demonstrating a benefit of rivaroxaban over aspirin when 74% of the anticipated efficacy events had occurred. The median follow-up duration was 11 months. The rivaroxaban and aspirin groups did not differ in the risks of first recurrent stroke or systemic embolism (primary efficacy outcome) (5.1%/year vs. 4.8%/year; hazard ratio [HR], 1.07; 95% confidence interval [CI], 0.87 to 1.33; P=0.52) and recurrent ischemic stroke (4.7%/year vs. 4.7%/year; HR, 1.01; 95% CI, 0.81 to 1.26). However, rivaroxaban vs. aspirin had more major bleedings (1.8%/year vs. 0.7%/year; HR, 2.72; 95% CI, 1.68 to 4.39; P<0.001) and more symptomatic intracranial hemorrhages (0.6%/year vs. 0.1%/year; HR, 4.02; 95% CI, 1.51 to 10.7; P=0.003) [13].
Arterial embolism from carotid plaque or mild carotid stenosis (<50%) might be better prevented with anticoagulation than with antiplatelet. Among patients in NAVIGATE-ESUS, 2,905 (40%) had carotid plaque, and 490 (11%) had mild carotid stenosis. Rivaroxaban was not superior to aspirin for preventing recurrent ischemic stroke in patients with carotid plaque (5.9%/year vs. 4.9%/year; HR, 1.20; 95% CI, 0.86 to 1.68) and in those with mild carotid stenosis (5.0%/year vs. 5.9%/year; HR, 0.85; 95% CI, 0.39 to 1.87). The risk of major bleeding was higher with rivaroxaban versus aspirin in these subgroups. About two-thirds of carotid plaques were present in the carotid artery ipsilateral to the index stroke and had a strong trend of higher risk of recurrent ischemic stroke, supporting that carotid plaque is an important culprit lesion in ESUS [17]. Therefore, future ESUS trials for NOACs might need to exclude patients for whom carotid atherosclerosis is likely a source of arterial embolism.
RE-SPECT ESUS (n=5,390) compared dabigatran (150 or 110 mg in age ≥75 or creatinine clearance [CrCl] 30 to 50 mL/min twice daily [BID]) and aspirin (100 mg OD). The median interval from stroke onset to randomization was 44 days and the median follow-up duration was 19 months. The risk of recurrent stroke (primary efficacy outcome) did not differ between the dabigatran and aspirin groups (4.1%/year vs. 4.8%/year; HR, 0.85; 95% CI, 0.69 to 1.03; P=0.10). Dabigatran versus aspirin was not superior for preventing recurrent ischemic stroke (4.0%/year vs. 4.7%/year; HR, 0.84; 95% CI, 0.68 to 1.03) and composite of stroke, myocardial infarction (MI), or vascular death (4.8%/year vs. 5.4%/year; HR, 0.88; 95% CI, 0.73 to 1.06). Dabigatran versus aspirin was not associated with more major bleedings (primary safety outcome) (1.7%/year vs. 1.4%/year; HR, 1.19; 95% CI, 0.85 to 1.66) or intracranial hemorrhages (0.7%/year vs. 0.7%/year; HR, 0.98; 95% CI, 0.60 to 1.60). However, there were more major or clinically relevant nonmajor (CRNM) bleedings with dabigatran (3.3%/year vs. 2.3%/year; HR, 1.44; 95% CI, 1.12 to 1.85). Of note, post hoc exploratory analysis suggested that there was no difference in the recurrent stroke risk during the first year, but the difference in favor of dabigatran was significant after 1 year. Increase in the prevalence of cardioembolic sources including undetected AF over time might account for the temporal pattern, but it was not systematically assessed [14].
Discrepancy in the results between NAVIGATE ESUS and RESPECT ESUS are noted. In NAVIGATE ESUS, rivaroxaban versus aspirin had more major bleeding and intracranial hemorrhage. However, in RE-SPECT ESUS, there was no significant differences between dabigatran and aspirin in the risks of major bleeding and intracranial hemorrhage. On the other hand, the risk of recurrent ischemic stroke was almost identical between rivaroxaban and aspirin in NAVIGATE ESUS, but was nonsignificantly lower with dabigatran than with aspirin in RE-SPECT ESUS. The observation suggests that dabigatran might be better than rivaroxaban, but indirect comparison limits valid interpretation.
The two ESUS trials showed that neither rivaroxaban nor dabigatran was more effective than aspirin in patients selected with the current ESUS criteria. The heterogeneity of stroke mechanisms in ESUS likely accounts for the neutral results. Covert AF, which benefits most from NOACs, appears less presented in the two trials. During the follow-up, AF was newly detected in only 2% of the NAVIGATE ESUS population and 0.8% in the RE-SPECT ESUS population. Therefore, for future trials, the current broad ESUS concept should be modified to reliably identify patients who likely benefit from NOACs.
ARCARDIA and ATTICUS are ongoing to compare apixaban and aspirin in more selective patients who have a high risk of cardiac embolism [15,16]. ARCADIA is enrolling patients with ESUS and atrial cardiopathy (P-wave terminal force >5,000 μV×ms on electrocardiogram, serum N-terminal prohormone of brain natriuretic peptide >250 pg/mL, or left atrial diameter index ≥3 cm/m2 on echocardiogram). The primary endpoint is recurrent stroke during at least 18-month follow-up, and the estimated sample size is 1,100 [15]. ATTICUS (n=500 planned) is enrolling patients with acute stroke (<7 days from onset), implanted insertable cardiac monitor, and potential risks of cardioembolism (left atrium size >45 mm, spontaneous echogenic contrast in left atrial appendage, flow velocity in left atrial appendage ≤0.2 m/sec, atrial high rate episodes, patent foramen ovale [PFO], or CHA2DS2-VASc score ≥4) to assess new ischemic lesions on follow-up MRI at 12 months [16].
Patent foramen ovale
No RCT has specifically assessed NOACs in PFO. In CLOSE (Patent Foramen Ovale Closure or Anticoagulants versus Antiplatelet Therapy to Prevent Stroke Recurrence), oral anticoagulation versus antiplatelet had a trend toward superiority for preventing recurrent stroke (HR, 0.43; 95% CI, 0.1 to 1.5; P=0.17). However, only 13 among 187 patients (7.0%) in the anticoagulation group received NOACs, which did not allow adequate analysis [18].
Patients with PFO were included in NAVIGATE ESUS and RESPECT ESUS. In NAVIGATE ESUS, 534 patients (7.4%; 259 randomized to rivaroxaban and 275 to aspirin) had PFO [13]. In this PFO subgroup, the risk of recurrent ischemic stroke was lower with rivaroxaban than with aspirin, but was not statistically different (HR, 0.54; 95% CI, 0.22 to 1.36]). In addition, the treatment effect was not modified by the presence of PFO (P for interaction=0.18) [19]. In RE-SPECT ESUS, 680 patients (12.6%; 319 in the dabigatran group and 361 in the aspirin group) had PFO. The risk of recurrent stroke was comparable between patients on dabigatran and those on aspirin (5.0%/year vs. 5.3%/year; HR, 0.88; 95% CI, 0.45 to 1.71), and the treatment effect was consistent between patients with PFO and those without PFO [14].
Therefore, the current available evidence does not support NOACs over aspirin in patients with ESUS and PFO. A metaanalysis combining data from NAVIGATE ESUS, PFO in Cryptogenic Stroke Study (PICSS), and CLOSE suggests that anticoagulation (warfarin and NOACs combined) versus aspirin in patients with cryptogenic stroke and PFO might be better for preventing recurrent ischemic stroke (odds ratio, 0.48; 95% CI, 0.24 to 0.96; P=0.04) without heterogeneity across the trials (I2=0%) [19]. Future trials are needed to determine the role of NOACs in cryptogenic stroke and PFO; NOACs versus aspirin in low risk PFO and NOACs versus PFO closure in high risk PFO.
Cerebral venous thrombosis
The standard therapy in patients with cerebral venous thrombosis (CVT) is parenteral anticoagulation in acute phase and then warfarin for 3 to 12 months or lifelong according to underlying conditions [20]. NOACs are promising options for long-term oral anticoagulation, but only data from small sized observational studies are available [21-24]. The 2017 European Stroke Organization guidelines do not recommend NOACs for the treatment of CVT, especially during the acute phase because of the very low quality of evidence [25]. Recently, the results of RE-SPECT CVT (n=120), an exploratory phase III trial to compare dabigatran and warfarin (parenteral heparin for 5 to 15 days in both treatments) [26], were presented at the 2018 World Stroke Congress. There were neither thrombotic event nor death in the two groups for up to 24 weeks. Major bleeding events occurred in one patient with dabigatran and two with warfarin. More data from large RCTs are needed to change clinical practice.
Noncardioembolic stroke
Large phase III trials have evaluated NOACs in non-AF patients with ACS or with stable atherosclerotic vascular disease, but there has been no large RCT in noncardioembolic ischemic stroke. In a small single arm trial (n=53), dabigatran initiated within 24 hours from stroke onset and continued for 30 days was safe without symptomatic hemorrhagic transformation in patients with minor stroke (National Institutes of Health Stroke Scale [NIHSS] score ≤3) [27].
Dabigatran Following Transient Ischemic Attack and Minor Stroke (DATAS) II (n=300), a phase II trial, compared dabigatran (150 or 110 mg for age >80 or glomerular filtration rate 30 to 50 mL/min BID) and aspirin (325 mg loading and 81 mg OD) for 30 days in patients with noncardioembolic transient ischemic attack or acute minor ischemic stroke (NIHSS score ≤9 and lesion volume on diffusion-weighted imaging <25 mL) within 72 hours of symptom onset [28]. The results presented at the 2018 European Stroke Organization Conference showed that there were no symptomatic hemorrhagic transformation (primary outcome) in both the groups. On follow-up MRI, dabigatran versus aspirin nonsignificantly had more asymptomatic hemorrhagic transformations (7.8% vs. 3.5%; relative risk [RR], 2.2; 95% CI, 0.79 to 6.21) and less recurrent ischemic lesions (6.3% vs. 9.9%; RR, 0.64; 95% CI, 0.29 to 1.44).
Acute coronary syndrome without atrial fibrillation
In ACS, activations of platelet and coagulation pathways are main mechanisms of thrombogenesis, and thereby combination therapy with anticoagulation and antiplatelet is more effective to prevent ischemic events than antiplatelet alone. The current guidelines recommend parenteral anticoagulation in addition to dual antiplatelet therapy (DAPT) during acute hospitalization or percutaneous coronary intervention (PCI) [29,30]. After the acute period, DAPT is the current standard of care, but the residual risk of major ischemic vascular events remains high up to 1 year. Studies showed that the formation of thrombin, a key factor in platelet activation and coagulation, remained increased after the acute period, which was associated with an increased risk of cardiac events [31-33]. In this context, NOACs on top of DAPT have been investigated in ACS without AF.
In RandomizEd Dabigatran Etexilate Dose Finding Study in Patients With Acute Coronary Syndromes Post Index Event With Additional Risk Factors for Cardiovascular Complications Also Receiving Aspirin and Clopidogrel: Multi-centre, Prospective, Placebo Controlled, Cohort Dose Escalation Study (REDEEM; n=1,861), a phase II dose-finding trial, dabigatran (50 to 150 mg BID) compared to placebo increased the risk of major or CRNM bleeding in a dose-dependent manner. D-dimer levels measured as an efficacy indicator were reduced with all doses of dabigatran, but the reduction was not translated into clinical benefit [34].
In a phase II dose-selection trial (Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Standard Therapy in Subjects with Acute Coronary Syndrome–Thrombolysis in Myocardial Infarction [ATLAS ACS-TIMI 46], n=3,491), rivaroxaban (at doses 5 to 20 mg OD) compared to placebo increased the risk of clinically significant bleeding in a dose-dependent manner, but reduced composite of death, MI, or stroke [35]. Based on these findings, ATLAS ACS 2-TIMI 51, a phase III trial (n=15,526), compared rivaroxaban 2.5 and 5 mg BID versus placebo. Composite of vascular death, MI, or stroke (primary efficacy outcome) was reduced with both the 2.5 mg dose BID (9.1% vs. 10.7%; HR, 0.84; 95% CI, 0.72 to 0.97; P=0.02) and the 5 mg dose BID (8.8% vs. 10.7%; HR, 0.85; 95% CI, 0.73 to 0.98; P=0.03) compared to placebo. Benefit on vascular death was seen with the 2.5 mg dose BID versus placebo (2.7% vs. 4.1%; HR, 0.66; 95% CI, 0.51 to 0.86; P=0.002), but not with the 5 mg BID dose. However, rivaroxaban versus placebo significantly increased the risk of major bleeding not associated with coronary artery bypass grafting (CABG) (2.1% [both rivaroxaban doses combined] vs. 0.6%; HR, 3.96; 95% CI, 2.46 to 6.38; P<0.001), and the higher dose regimen had a higher bleeding risk than did the lower dose regimen (2.4% vs. 1.8%) [36].
In a dose-finding trial (Apixaban added to DAPT was evaluated in the Apixaban for Prevention of Acute Ischemic Events [APPRAISE], n=1,715), apixaban (from 2.5 mg BID to 20 mg OD) compared to placebo had a dose-dependent increase in the risk of clinically significant bleeding, but had a trend toward benefit for reducing ischemic events [37]. However, in the phase III APPRAISE-2 trial (n=7,392), apixaban 5 mg (2.5 mg with CrCl <40 mL/min) BID compared to placebo significantly increased the risk of major bleeding (2.4%/year vs. 0.9%/year; HR, 2.59; 95% CI, 1.50 to 4.46; P=0.001), and did not reduce composite of vascular death, MI, or ischemic stroke (13.2%/year vs. 14.0%/year; HR, 0.95; 95% CI, 0.80 to 1.11; P=0.51) [38].
In the phase III trials in ACS without AF, NOACs added to DAPT were associated with a higher risk of major bleeding. However, the European Medicines Agency approved the use of rivaroxaban 2.5 mg BID added to standard antiplatelet therapy because ATLAS ACS 2-TIMI 51 showed benefits of low dose rivaroxaban regarding major ischemic events and vascular death. The European Society of Cardiology guidelines support this regimen in ACS patients with a low bleeding risk [29]. However, the U.S. Food and Drug Administration did not approve the use of rivaroxaban in ACS without AF.
In contrast to earlier RCTs investigating NOACs added to DAPT, Randomized, Double-Blind, Double-Dummy, Active-controlled, Parallel-group, Multicenter Study to Compare the Safety of Rivaroxaban Versus Acetylsalicylic Acid in Addition to Either Clopidogrel or Ticagrelor Therapy in Subjects With Acute Coronary Syndrome (GEMINI-ACS-1; n=3,037) compared the safety of dual regimen with low-dose rivaroxaban (2.5 mg BID) plus P2Y12 inhibitor and aspirin plus P2Y12 inhibitor. During a median follow-up of 291 days, the rate of clinically significant bleeding not related to CABG was similar between the rivaroxaban and aspirin groups (5% vs. 5%; HR, 1.09; 95% CI, 0.80 to 1.50; P=0.584). The risk of composite of vascular death, MI, stroke, or definite stent thrombosis (exploratory ischemic endpoint) was comparable between the rivaroxaban and aspirin groups (5% vs. 5%; HR, 1.06; 95% CI, 0.77 to 1.46; P=0.732) [39]. GEMINIACS-1 showed that dual therapy with NOAC plus P2Y12 inhibitor can be as safe as the standard DAPT. However, it was a phase II trial and not powered to fully assess the efficacy.
Stable atherosclerotic vascular disease
Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS; n=27,395) compared combination therapy of rivaroxaban (2.5 mg BID) plus aspirin (100 mg OD), rivaroxaban alone (5 mg BID), and aspirin alone (100 mg OD) in patients with stable atherosclerotic vascular disease (coronary artery disease or peripheral arterial disease) [40]. COMPASS was early terminated because of the superior efficacy of combination therapy over aspirin alone at the time of the first interim analysis when 50% of planned events had occurred. Combination therapy versus aspirin alone significantly reduced composite of cardiovascular death, stroke, or MI (primary efficacy outcome) during a mean follow-up of 23 months (4.1% versus 5.4%; HR, 0.76; 95% CI, 0.66 to 0.86; P<0.001). The effect was consistent across diverse subgroups. Combination therapy versus aspirin alone was associated with a higher risk of major bleeding (3.1% vs. 1.9%; HR, 1.70; 95% CI, 1.40 to 2.05; P<0.001), which was mainly driven by gastrointestinal (G-I) bleedings. The composite net-clinical-benefit outcome (cardiovascular death, stroke, MI, fatal bleeding, or symptomatic bleeding into a critical organ) occurred less with combination therapy than with aspirin alone (4.7% vs. 5.9%; HR, 0.80; 95% CI, 0.70 to 0.91; P<0.001). All-cause death and vascular death were lower with combination therapy compared to aspirin alone. However, rivaroxaban 5 mg BID alone had no significant benefit over aspirin alone in the primary efficacy outcome (4.9% vs. 5.4%; HR, 0.90; 95% CI, 0.79 to 1.03; P=0.12) and in the composite net-clinical-benefit outcome (5.5% vs. 5.9%; HR, 0.94; 95% CI, 0.84 to 1.07; P=0.36), but increased the risk of major bleeding (2.8% vs. 1.9%; HR, 1.51; 95% CI, 1.25 to 1.84; P<0.001).
Based on the COMPASS results, the U.S. Food and Drug Administration and the European Medicines Agency approved the 2.5 mg BID dose of rivaroxaban in combination with aspirin in patients with stable coronary artery disease or peripheral arterial disease. In a subgroup analysis of patients with peripheral arterial disease, the benefit of combination therapy in patients with carotid stenosis >50% or a history of carotid revascularization was consistent with those without carotid disease [41]. However, COMPASS excluded patients with recent stroke, prior history of lacunar infarction, or prior history of intracerebral hemorrhage, and only 3.8% of the enrolled patients had a history of prior stroke [40], which limits the application of the trial findings to stroke population.
Acute coronary syndrome with atrial fibrillation
In AF patients with ACS, DAPT is indicated for ACS and long-term oral anticoagulation is indicated for AF. Triple therapy with oral anticoagulation plus DAPT for 1 to 6 months is the standard antithrombotic therapy, but inevitably increases the risk of major bleeding. RCTs compared NOACs in combination with DAPT or P2Y12 inhibitor monotherapy vs. triple therapy with warfarin plus DAPT, primarily focusing on the risk of major bleedings rather than ischemic events.
Open-Label, Randomized, Controlled, Multicenter Study Exploring Two Treatment Strategies of Rivaroxaban, and a Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy in Subjects with Atrial Fibrillation who Undergo Percutaneous Coronary Intervention (PIONEER AF-PCI; n=2,124) compared rivaroxaban 15 mg OD plus P2Y12 inhibitor, rivaroxaban 2.5 mg BID plus DAPT, and warfarin plus DAPT in patients with AF undergoing PCI [42]. Both the rivaroxaban-based regimens compared to warfarin plus DAPT reduced the risk of clinically-significant bleeding (primary endpoint) at 12 months: rivaroxaban 15 mg OD plus P2Y12 inhibitor (16.8% vs. 26.7%; HR, 0.59; 95% CI, 0.47 to 0.76; P<0.001) and rivaroxaban 2.5 mg BID plus DAPT (18.0% vs. 26.7%; HR, 0.63; 95% CI, 0.50 to 0.80; P<0.001). The rate of composite of vascular death, MI, or stroke did not differ across the three groups.
Randomized Evaluation of Dual Antithrombotic Therapy With Dabigatran vs. Triple Therapy With Warfarin in Patients With Nonvalvular Atrial Fibrillation Undergoing Percutaneous Coronary Intervention (RE-DUAL PCI; n=2,725) compared two dabigatran regimens (110 and 150 mg BID) plus P2Y12 inhibitor monotherapy versus triple therapy with warfarin plus DAPT in patients with AF undergoing PCI [43]. The primary endpoint of major or CRNM bleeding during a mean follow-up of 14 months was lower in the 110 mg dabigatran plus P2Y12 inhibitor group than in the warfarin-based triple therapy group (15.4% vs. 26.9%; HR, 0.52; 95% CI, 0.42 to 0.63; P<0.001 for noninferiority; P<0.001 for superiority). The 150 mg dabigatran plus P2Y12 inhibitor group also had a lower bleeding risk compared to the corresponding warfarin-based triple therapy group (20.2% vs. 25.7%; HR, 0.72; 95% CI, 0.58 to 0.88; P<0.001 for noninferiority; P=0.002 for superiority). Dabigatran-based dual therapy (two regimens combined) was not inferior to warfarinbased triple therapy for the composite efficacy endpoint of thromboembolic events (MI, stroke, or systemic embolism), death, or unplanned revascularization (13.7% vs. 13.4%; HR, 1.04; 95% CI, 0.84 to 1.29; P=0.005 for noninferiority). However, the sample size was not powered to adequately prove the noninferiority of each dabigatran regimen to triple therapy for the composite efficacy endpoint.
An Open-label, 2×2 Factorial, Randomized Controlled, Clinical Trial to Evaluate the Safety of Apixaban vs. Vitamin K Antagonist and Aspirin vs. Aspirin Placebo in Patients With Atrial Fibrillation and Acute Coronary Syndrome or Percutaneous Coronary Intervention (AUGUSTUS; n=4,614) was a 2×2 factorial trial (open-label comparison of apixaban 5 mg [or 2.5 mg if dose reduction is indicated] BID vs. warfarin and blinded comparison of aspirin vs. matching placebo) to assess the noninferiority of apixaban to warfarin and the superiority of placebo to aspirin for the primary outcome of major or CRNM bleeding in AF patients with ACS or PCI [44]. All patients received an approved P2Y12 inhibitor. The risk of major or CRNM bleeding for 6 months was lower with apixaban than with warfarin (10.5% vs. 14.7%; HR, 0.69; 95% CI, 0.58 to 0.81; P<0.001 for both noninferiority and superiority) and higher with aspirin versus placebo (16.1% vs. 9.0%; HR, 1.89; 95% CI, 1.59 to 2.24; P<0.001). When the interventions were combined, the risk was highest with warfarin plus aspirin (18.7%), followed by apixaban plus aspirin (13.8%), warfarin alone (10.9%), and apixaban alone (7.3%). The risk of death or hospitalization was lower with apixaban versus warfarin (23.5% vs. 27.4%; HR, 0.83; 95% CI, 0.74 to 0.93; P=0.002 for superiority), but was similar with aspirin versus placebo (26.2% vs. 24.7%; HR, 1.08; 95% CI, 0.96 to 1.21). It was highest with warfarin plus aspirin (27.5%) and lowest with apixaban alone (22.0%). Another efficacy outcome of death or ischemic event did not differ between apixaban and warfarin and between aspirin and placebo. Overall, apixaban plus P2Y12 inhibitor monotherapy was the most favored regimen.
PIONEER AF-PCI, RE-DUAL PCI, and AUGUSTUS demonstrated the superiority of NOAC over warfarin for the risk of clinically significant bleeding in AF patients with ACS or PCI. The 2018 Joint European consensus document states that, as part of triple or dual therapy, NOACs are preferred options over oral vitamin K antagonist (VKA) in AF patients with ACS and/or undergoing PCI [45]. The 2019 updated American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines recommend NOACs, but there is no statement with regard to the preference of NOACs over warfarin [6]. NOAC plus P2Y12 inhibitor monotherapy compared to NOAC-based or warfarin-based triple therapy does not appear to increase the risk of ischemic event. However, it should be noted that all the trials were not designed to primarily assess the efficacy of ischemic event outcome. Edoxaban Treatment vs. Vitamin K Antagonist in Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention (ENTRUST-AF-PCI; n=1,500 planned) is ongoing to compare endoxaban plus P2Y12 inhibitor and warfarin-based triple therapy (aspirin use for 1 to 12 months), primarily focusing on the risk of major or CRNM bleeding [46].
Chronic heart failure
CHF, even with sinus rhythm, is associated with activation of thrombin-related pathways and increases the risk of thromboembolism. However, individual RCTs failed to show the benefit of warfarin over aspirin, and a meta-analysis indicated that warfarin versus aspirin was associated with lower risks of any stroke and ischemic stroke, but the benefit was offset by an increased risk of major bleeding [47]. Given their better safety profile compared to warfarin, NOACs are promising options.
Study to Assess the Effectiveness and Safety of Rivaroxaban in Reducing the Risk of Death, Myocardial Infarction, or Stroke in Participants with Heart Failure and Coronary Artery Disease Following an Episode of Decompensated Heart Failure (COMMANDER-HF; n=5,022) compared rivaroxaban 2.5 mg BID and placebo in addition to standard care (98.5% of patients received single antiplatelet or DAPT) in CHF patients with a recent episode of worsening heart failure and coronary heart disease. During a median follow-up of 21 months, the rivaroxaban and placebo groups did not differ in the composite of all-cause death, MI, or stroke (25.0% vs. 26.2%; HR, 0.94; 95% CI, 0.84 to 1.05; P=0.27) and the principal safety outcome of fatal bleeding or bleeding into a critical space (0.7% vs. 0.9%; HR, 0.80; 95% CI, 0.43 to 1.49; P=0.48). Heart failure-related deaths rather than thromboembolic events constituted a large proportion of clinical events, which likely accounts for the lack of anticoagulation benefit in this study population [48]. In a post hoc analysis, a newly defined thromboembolic composite (MI, ischemic stroke, sudden/unwitnessed death, symptomatic VTE) was lower with rivaroxaban than with placebo [49]. Therefore, future NOAC trials in CHF should be designed to target thromboembolic events.
Deep vein thrombosis prophylaxis after hip or knee arthroplasty
Patients undergoing total hip arthroplasty (THA) or total knee arthroplasty (TKA) are at high risk of VTE including deep vein thrombosis (DVT) and pulmonary embolism (PE), and anticoagulation therapy during the high risk period is recommended [50].
Four RCTs compared dabigatran (150 mg or 220 mg OD) and enoxaparin (40 mg OD or 30 mg BID) in patients undergoing THA or TKA (Table 1) [7,8,51,52]. Three trials showed non-inferiority of dabigatran to enoxaparin for preventing VTE or all-cause death [7,8,52]. A pooled analysis demonstrated that dabigatran 220 mg OD and 150 mg OD versus enoxaparin were comparable for preventing major VTE or VTE-related death (3.0% and 3.8% vs. 3.3%) and for the risk of major bleeding (1.4% and 1.1% vs. 1.4%) [53].
The REgulation of Coagulation in ORthopedic Surgery to Prevent DVT and PE, Controlled, Double-blind, Randomized Study of BAY 59-7939 in the Extended Prevention of VTE in Patients Undergoing Elective Total Hip Replacement (RECORD) 1 (n=4,541), 2 (n=2,509), 3 (n=2,531), and 4 (n=3,148) trials compared rivaroxaban (10 mg OD) and enoxaparin (40 mg OD or 30 mg BID) in patients undergoing THA or TKA [9-11,54]. All the RECORD trials showed the superior efficacy of rivaroxaban over enoxaparin for preventing DVT, nonfatal PE, or all-cause death without increasing major bleeding events. In a pooled analysis, rivaroxaban versus enoxaparin significantly reduced the composite of symptomatic VTE and all-cause death (0.6% vs. 1.3%; HR, 0.42; 95% CI, 0.29 to 0.63), and did not significantly increase the risk of major or CRNM (2.8% vs. 2.5%, P=0.19) [55].
The Apixaban Dose Orally vs. Anticoagulation with Enoxaparin (ADVANCE) 1 (n=3,195), 2 (n=3,057), 3 (n=3,866) trials compared apixaban (2.5 mg BID) versus enoxaparin (30 mg BID or 40 mg OD) in patients with TKA or THA [56-58]. Compared to enoxaparin 30 mg BID, apixaban was not noninferior, albeit comparable, for the risk of total VTE or all-cause death, but had less major or CRNM bleeding [56]. When compared to exoxaparin 40 mg OD, apixaban was more effective for preventing total VTE or all-cause death without a significant increase in the risk of major or CRNM bleeding [57,58].
In two trials (Studying Thrombosis After Replacement Surgery [STARS] E-3 [n=716] and STARS J-V [n=610]) enrolling East Asian patients with TKA or THA, edoxaban 30 mg OD versus enoxaparin 20 mg BID was more effective for preventing symptomatic PE or asymptomatic and symptomatic DVT without significantly increasing major or CRNM bleedings for 11 to 14 days [59,60]. In a pooled analysis, edoxaban versus enoxaparin had a lower VTE risk (5.1% vs. 10.7%, P<0.001) and a similar major or CRNM bleeding risk (4.6% vs. 3.7%, P=0.427) [61].
The 9th American College of Chest Physicians guidelines recommend thromboprophylaxis for a minimum of 10 to 14 days and extension in the outpatient period up to 35 days after surgery in patients undergoing THA or TKA. For anticoagulation therapy, use of dabigatran, rivaroxaban, or apixaban as well as low-molecular weight heparin (LMWH), fondaparinux, or VKA is recommended [50].
Acute venous thromboembolism
VTE is a serious life-threatening condition, and anticoagulation is the mainstay for acute management and long-term prevention of recurrent VTE. RCTs in patients with VTE have focused to demonstrate better safety and similar efficacy of NOACs compared to conventional anticoagulation therapy of parenteral anticoagulation switched to warfarin.
In Phase III, Randomised, Double Blind, Parallel-group Study of the Efficacy and Safety of Oral Dabigatran Etexilate 150 mg Twice Daily Compared to Warfarin (INR 2.0–3.0) for 6 Month Treatment of Acute Symptomatic Venous Thromboembolism, Following Initial Treatment (5–10 Days) With a Parenteral Anticoagulant Approved for This Indication (RE-COVER; n=2,564) and RE-COVER II (n=2,589), dabigatran 150 mg BID versus warfarin (parenteral anticoagulation at least 5 days in both treatments) was not inferior for preventing recurrent VTE or VTE-related death, and had a comparable risk of major bleeding for 6 months in patients with acute, symptomatic proximal DVT or PE [62,63]. In a pooled analysis, dabigatran versus warfarin was comparable for preventing recurrent VTE or VTE-related death (2.4% vs. 2.2%; HR, 1.09; 95% CI, 0.76 to 1.57) and for the risk of major bleeding (1.4% vs. 2.0%; HR, 0.73; 95% CI, 0.48 to 1.11), but had less major or CRNM bleedings (5.3% vs. 8.5%; HR, 0.62; 95% CI, 0.50 to 0.76) [63].
Twice-daily Oral Direct Thrombin Inhibitor Dabigatran Etexilate in the Long-term Prevention of Recurrent Symptomatic Proximal Venous Thromboembolism in Patients With Symptomatic Deep-vein Thrombosis or Pulmonary Embolism (RESONATE; placebo-control study, n=1,353) and Twice-daily Oral Direct Thrombin Inhibitor Dabigatran Etexilate in the Long Term Prevention of Recurrent Symptomatic VTE (RE-MEDY; active-control study with warfarin, n=2,866) evaluated the extended use of dabigatran 150 mg BID for 3 to 36 months in patients who had already completed 3-month anticoagulation therapy [64]. Dabigatran versus placebo significantly reduced the risk of recurrent symptomatic VTE (0.4% vs. 5.6%; HR, 0.08; 95% CI, 0.02 to 0.25; P<0.001), but was associated with more major or CRNM bleedings (5.3% vs. 1.8%; HR, 2.92; 95% CI, 1.52 to 5.60; P=0.001). When compared to warfarin, dabigatran was comparable for preventing recurrent symptomatic VTE (1.8% vs. 1.3%; HR, 1.44; 95% CI, 0.78 to 2.64), but had a lower risk of major or CRNM bleeding (5.6% vs. 10.2%; HR, 0.54; 95% CI, 0.41 to 0.71; P<0.001).
In Oral Direct Factor Xa Inhibitor Rivaroxaban in Patients With Acute Symptomatic Deep-Vein Thrombosis or Pulmonary Embolism (EINSTEIN)-DVT (n=3,449, patients with acute symptomatic DVT) and EINSTEIN-PE (n=4,832, patients with acute symptomatic PE) [65,66], rivaroxaban alone (15 mg BID for 3 weeks and then 20 mg OD without enoxaparin bridging) versus warfarin bridging with enoxaparin was not inferior for preventing recurrent symptomatic VTE (EINSTEIN-DVT: 2.1% vs. 3.0%; HR, 0.68; 95% CI, 0.44 to 1.04; P<0.001 for noninferiority; and EINSTEIN-PE: 2.1% vs. 1.8%; HR, 1.12; 95% CI, 0.75 to 1.68; P=0.003 for noninferiority), and was comparable for the risk of major or CRNM bleeding (EINSTEIN-DVT: 8.1% vs. 8.1%; HR, 0.97; 95% CI, 0.76 to 1.22; P=0.77; and EINSTEIN-PE: 10.3% vs. 11.4%; HR, 0.90; 95% CI, 0.76 to 1.07; P=0.23). In EINSTEIN-PE, major bleedings were less frequent with rivaroxaban (1.1% vs. 2.2%; HR, 0.49; 95% CI, 0.31 to 0.79; P=0.003).
EINSTEIN–extension (n=1,197) assessed the extended use of rivaroxaban 20 mg OD for 6 or 12 months in patients with prior anticoagulation therapy for 6 to 12 months after acute symptomatic DVT or PE and equipoise for continued anticoagulation [65]. Rivaroxaban versus placebo significantly reduced the risk of recurrent VTE (1.3% vs. 7.1%; HR, 0.18; 95% CI, 0.09 to 0.39; P<0.001). The increased risk with rivaroxaban was not significant for major bleedings (0.7% vs. 0%, P=0.11), but was significant for major or CRNM bleeding (6.0% vs. 1.2%; HR, 5.19; 95% CI, 2.3 to 11.7; P<0.001).
In Apixaban for the Initial Management of Pulmonary Embolism and Deep-Vein Thrombosis as First-Line Therapy (AMPLIFY; n=5,244), apixaban alone (10 mg BID for 7 days followed by 5 mg BID for 6 months) versus warfarin bridging with enoxaparin in patients with acute VTE was not inferior for preventing recurrent symptomatic VTE (2.3% vs. 2.7%; RR, 0.84; 95% CI, 0.60 to 1.18; P<0.001 for noninferiority), and had a lower risk of major bleeding (0.6% vs. 1.8%; RR, 0.31; 95% CI, 0.17 to 0.55; P<0.001) [67]. In AMPLIFY-EXT (n=2,486) enrolling patients with prior anticoagulation for 6 to 12 months and equipoise for continued anticoagulation, two apixaban regimens (apixaban 5 mg BID and 2.5 mg BID) versus placebo for 12 months were superior for preventing recurrent VTE or all-cause death (5 mg regimen: 4.2% vs. 11.6%; RR, 0.36; 95% CI, 0.25 to 0.53; P<0.001; and 2.5 mg regimen: 3.8% vs. 11.6%; RR, 0.33; 95% CI, 0.22 to 0.48; P<0.001). The three groups had comparable risks of major bleeding (0.1% vs. 0.2% vs. 0.5%) and major or CRNM bleeding (4.3% vs. 3.2% vs. 2.7%) [68].
In Phase 3, Randomized, Parallel-Group, Multi-Center, Multi-National Study for the Evaluation of Efficacy and Safety of Heparin/Edoxaban Versus Heparin/Warfarin in Subjects With Symptomatic Deep-Vein Thrombosis and or Pulmonary Embolism (Hokusai-VTE; n=8,292), edoxaban 60 mg (30 mg for CrCL 30 to 50 mL/min or body weight <60 kg) OD versus warfarin (parenteral anticoagulation at least 5 days in both treatments) for 3 to 12 months in patients with acute symptomatic DVT or PE was not inferior for preventing symptomatic recurrent VTE (3.2% vs. 3.5%; HR, 0.89; 95% CI, 0.70 to 1.13; P<0.001 for noninferiority), and had a lower risk of major or CRNM bleeding (8.5% vs. 10.3%; HR, 0.81; 95% CI, 0.71 to 0.94; P=0.004 for superiority) [69].
RCTs in acute VTE demonstrated that NOACs compared to conventional anticoagulation strategy had a similar efficacy and a comparable or better safety. Extended use of NOACs reduced recurrent VTE at the cost of more major bleedings compared to placebo, and reduced the risk of major bleeding with a similar efficacy compared to warfarin. The American College of Chest Physicians and European Society of Cardiology guidelines recommend NOACs over warfarin in patients with VTE not associated with cancer [70,71].
Venous thromboembolism related to active cancer
Patients with cancer have a 7-fold increased risk of VTE compared to individuals without cancer [72]. RCTs showed that LMWHs were more effective than and as safe as VKAs. Despite of their convenient administration and predictable effects, NOACs in cancer patients have disadvantages of (1) limited evidence from RCTs, (2) absorption affected by vomiting, a common symptom in cancer patients, and (3) drug interactions with chemotherapy agents. The 2016 CHEST guidelines recommend extended anticoagulant therapy (no scheduled stop date) over 3 months and suggest LMWHs over NOACs for anticoagulation therapy [70]. After then, several RTCs have been published.
Hokusai-VTE cancer (n=1,050) in patients with cancer and acute symptomatic or incidentally detected VTE showed that edoxaban (60 mg OD after LMWH for at least 5 days) versus dalteparin (200 IU/kg OD for 1 month followed by 150 IU/kg OD) was not inferior for preventing recurrent VTE or major bleeding during 12 months (12.8% vs. 13.5%; HR, 0.97; 95% CI, 0.70 to 1.36; P=0.006 for noninferiority; P=0.87 for superiority): recurrent VTEs (7.9% vs. 11.3%; HR, 0.71; 95% CI, 0.48 to 1.06; P=0.09) and major bleedings (6.9% vs. 4.0%; HR, 1.77; 95% CI, 1.03 to 3.04; P=0.04). More major bleedings with edoxaban was caused by more G-I bleedings in G-I cancer patients [73].
In Anticoagulation therapy in SELECTeD cancer patients at risk of recurrence of venous thromboembolism (SELECT-D; n=406), rivaroxaban (15 mg BID for 3 weeks and then 20 mg OD) versus dalteparin (200 IU/kg daily for 1 month and then 150 IU/kg daily) in patients with active cancer and VTE reduced the risk of recurrent VTE (4% vs. 11%; HR, 0.43; 95% CI, 0.19 to 0.99), but increased major bleeding events nonsignificantly (6% vs. 4%; HR, 1.83; 95% CI, 0.68 to 4.96) and CRNM significantly (13% vs. 4%; HR, 3.76; 95% CI, 1.63 to 8.69). The higher bleeding risk with rivaroxaban was largely driven by more G-I bleedings in G-I cancer patients [74].
In Apixaban and dalteparin in active malignancy associated venous thromboembolism (ADAM VTE; n=300), apixaban (10 mg BID for 7 days followed by 5 mg BID) versus dalteparin (200 IU/kg daily for 1 month followed by 150 IU/kg daily) in patients with active cancer and confirmed VTE was superior for preventing recurrent VTE for 6 months (3.4% vs. 14.1%; HR, 0.26; 95% CI, 0.09 to 0.80; P=0.0182). There were no major bleeding with apixaban and 3 (2.1%) with dalteparin [75,76].
In the 2018 updated National Comprehensive Cancer Network guidelines, edoxaban after parenteral anticoagulation for 5 to 10 days is newly listed as a category 1 recommendation. Apixaban monotherapy, rivaroxaban monotherapy, and dabigatran combined with at least 5 days of parenteral anticoagulation are recommended as alternatives for patients with compelling reasons to avoid LMWHs [77]. However, physicians should recognize the bleeding risk of NOCAs in patients with G-I cancer and the effect of vomiting and chemotherapeutic agents on the effect of NOACs.
Acute medical illness at high risk of venous thromboembolism
Patients who are hospitalized and immobilized for acute medical illness are at increased risk of VTE. Low dose parenteral anticoagulation for 6 to 14 days is recommended by guidelines [78]. The risk of VTE persists during the first month after discharge, but earlier RCTs failed to show the benefit of extended use of enoxaparin, rivaroxaban, or apixaban [79-81]. Recently, in Acute Medically Ill VTE Prevention with Extended Duration Betrixaban (APEX; n=7,513) for patients with acute medical illness (acutely decompensated heart failure with symptomatic CHF, acute respiratory failure in patients with chronic symptomatic lung disease, acute infection without septic shock, acute rheumatic disorders, or acute ischemic stroke) and at high risk of VTE (reduced mobility and specific risk factors based on age, D-dimer level and history of either VTE or cancer), the extended use of betrixaban for 35 to 42 days (after enoxaparin for 6 to 14 days) vs. standard of care (enoxaparin for 6 to 14 days) reduced the risk of asymptomatic proximal DVT, symptomatic VTE, or VTE-related death (5.3% vs. 7.0%; RR, 0.76; 95% CI, 0.63 to 0.92; P=0.006) without increasing major bleeding events (0.7% vs. 0.6%; RR, 1.19; 95% CI, 0.67 to 2.12; P=0.55) [82]. Based on these results, betrixaban was approved for this indication by the U.S. Food and Drug Administration. In a subsequent analysis, the extended betrixaban use was associated with a lower risk of all stroke (0.54% vs. 0.97%; RR, 0.56; 95% CI, 0.32 to 0.96; P=0.032) and ischemic stroke (0.48% vs. 0.91%; RR, 0.53; 95% CI, 0.30 to 0.94; P=0.026) [83].
Antiphospholipid antibody syndrome
Antiphospholipid antibody (Ab) syndrome, diagnosed by at least one thrombotic events or pregnancy-related complications and at least two positive antiphospholipid Ab tests confirmed by follow-up tests, are at high risk of recurrent thrombotic event. The risk is highest in patients with positivity on all three Ab tests (lupus anticoagulant, anticardiolipin Ab, and anti–b2-glycoprotein I Ab), up to 6% to 13% per year. Trial on Rivaroxaban in AntiPhospholipid Syndrome (TRASP) compared rivaroxaban and warfarin in patients with a history of proven venous or arterial thrombosis and positive for all three antiphospholipid Ab tests. The trial was early terminated after enrolling 120 patients (64% with venous thrombosis, 21% with arterial thrombosis [13% with stroke], and 15% with venous and arterial thrombosis) because the rivaroxaban group versus the warfarin group had a higher risk of the composite of thromboembolic events, major bleeding, or vascular death (19% vs. 3%; HR, 6.7; 95% CI, 1.5 to 30.5) during a mean follow-up of 596 days. Seven thromboembolic events (four ischemic strokes and three MIs) occurred in the rivaroxaban group, but none in the warfarin group. Major bleeding was more common with rivaroxaban versus warfarin (4 events [7%] vs. 2 events [3%]) [84].
Conclusions
Large clinical trials have provided the evidential foundation for the use of NOACs in DVT prophylaxis with hip or knee arthroplasty, acute and long-term management of VTE with or without cancer, and concomitant ACS and AF. Although debate still remains, very low dose rivaroxaban added to standard antiplatelet therapy was beneficial in patients with ACS without AF and those with stable cardiovascular disease. Physicians should acknowledge the NOAC regimen with proven efficacy applicable to individual medical conditions. For secondary stroke prevention, the trial results, up to now, do not support the use of NOACs in ESUS. For other potential candidates of PFO-related stroke, acute ischemic stroke, and CVT, high quality evidence is lacking. Lessons from the earlier successful and unsuccessful trials can guide the optimal future trial design for NOACs in secondary stroke prevention.
Notes
Disclosure
Dr. Keun-Sik Hong has received lecture honoraria and research grants from Daiichi-Sankyo Korea, Pfizer Korea, Bristol-Myers Squibb Korea, Boehringer Ingelheim Korea, Bayer Korea from related to the current topic.