Depression after Subarachnoid Hemorrhage: A Systematic Review
Article information
Abstract
Background and Purpose
Depression is common and debilitating illness accompanying many neurological disorders including non-traumatic subarachnoid hemorrhage (SAH). The aim of this systematic review was to identify and critically appraise all published studies that have reported the frequency, severity and time course of depression after SAH, the factors associated with its development and the impact of depression on patients’ quality of life after SAH.
Methods
The PubMed database was searched for studies published in English that recruited at least 40 patients (>18 years old) after SAH who were also diagnosed with depression.
Results
Altogether 55 studies covering 6,327 patients met study entry criteria. The frequency of depression ranged from 0% to 61.7%, with a weighted proportion of 28.1%. Depression remained common even several years after the index SAH. Depression after SAH was associated with female sex, premorbid depression, anxiety, substance use disorders or any psychiatric disorders, and coping styles. Comorbid cognitive impairment, fatigue, and physical disability also increased the risk of depression. Aneurysmal SAH and infarction may be related to depression as well. Depression reduces the quality of life and life satisfaction in patients after SAH.
Conclusions
Depression is common after SAH and seems to persist. Further research is needed to clarify its time course and identify the neuroendocrine and neurochemical factors and brain circuits associated with the development of post-SAH depression. Randomized controlled treatment trials targeting SAH-related depression are warranted.
Introduction
Subarachnoid hemorrhage (SAH) is a relatively uncommon and severe type of stroke. As patients are affected by SAH at a mean age of 55 years, they can lose many years of productive life. The rupture of an intracranial aneurysm is the underlying cause in 85% of SAH cases [1]. Approximately 55% of patients survive SAH and regain independent functioning, whereas 19% remain dependent and 26% die [1]. Many survivors of SAH have long-term deficits in cognition, and decreased quality of life [2]. Neuropsychiatric disturbances such as depression, anxiety, post-traumatic stress disorder, and fatigue are not uncommon, yet often neglected in patients with SAH [3].
Depression is common in patients with neurological diseases such as Alzheimer’s disease, Parkinson’s disease, traumatic brain injury and stroke [4]. Depression is a frequent consequence of head injury, affecting up to 61% of patients. Depression is associated with worse global outcomes, impaired social functioning, difficulty performing activities of daily living, and a lower quality of life [5].
Post-stroke depression contributes to disability and increased mortality following stroke. Depression is increasingly becoming a standard part of post-stroke assessment and rehabilitation [6]. However, there is still a lack of methodically sound psychopharmacological and psychosocial treatment trials on SAH-related depression.
The aims of this systematic review were as follows: (1) to determine the frequency, severity and time course of depression after SAH; (2) to identify the factors associated with the development of depression after SAH, including patients’ demographic data, baseline characteristics of SAH, psychological factors including anxiety and cognitive impairment, somatic complications related to SAH (neuroendocrine changes, infarcts, and preexisting and post-SAH medical comorbidities); and (3) to evaluate the impact of depression on patients’ quality of life following SAH.
Methods
Literature search
This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline. The principal author (W.K.T.) searched the PubMed, EMBASE, PsycINFO, and Ovid Nursing databases on December 5, 2018, using the keywords “depression” or “mood” or “depressive” and “subarachnoid.” Two authors (W.K.T. and L.W.) read every title and abstract, obtained the full texts of potentially relevant papers, and applied inclusion and exclusion criteria to each text. Any uncertainties were discussed. W.K.T. also scrutinized the reference lists of included papers to identify further studies.
Inclusion criteria
Studies were included in the review if they (1) were written in English, (2) were published in peer-reviewed journals, (3) included 10 or more patients who had survived non-traumatic SAH and were older than 18 years [7], and (4) assessed patients for depression using a validated single or multiple item self-report instrument or diagnostic interview.
Exclusion criteria
Publications were excluded if they were (1) case reports, (2) pediatric studies (patients <18 years old), (3) dissertations, or (4) articles with no primary data (reviews, editorials letters, etc.), (5) sample size <40, (6) poor quality (a Strengthening the Report of Observational Studies in Epidemiology [STROBE] checklist score ≤13, i.e., 60% of maximum score).
Data extraction
Two authors (W.K.T. and L.W.) independently extracted the following data from the studies included in the review: study characteristics (aims/objectives, study design, inclusion and exclusion criteria, criteria for and measurement of depression), participants’ characteristics (definition of the study population, age, gender, number, ethnicity, and socio-economic status of the patients at the beginning and end of the study, the number of deaths due to SAH, drop-outs, and patients lost to follow-up before the end of study, first or recurrent SAH, severity of SAH, comorbidities and complications) and results (characteristics of patients’ subgroups, outcome data, and relationship between depression and patients’ characteristics or SAH characteristics and/or outcomes).
Quality assessment
We used STROBE statement for quality assessment of the included papers [8]. It consists of 22 items. We scored each item 1 point. The maximum possible score is 22.
Data synthesis
Statistical analyses were performed in Software R (package metaphor & meta, R Foundation for Statistical Computing, Vienna, Austria). The results are presented as a narrative review and are also tabulated. Frist, the weighted proportion of the frequency of depression was calculated. We conducted a metaanalysis of frequency of depression, using the variance-stablizing double-arscine method transformation [9]. Pooled estimates in both the overall (and subgroup) analyses were calculated using the Hartung-Knapp-Sidik-Jonkman method, under the random effect model [10]. Statistical heterogeneity among the trials was assessed, and P<0.1 was considered as statistical significance [11]. Level of heterogeneity was assessed by I2, which describes the percentage of total variation across studies because of heterogeneity rather than chance alone. A randomeffects model for the trials with statistically significant heterogeneity was used. Subgroup analyses were performed according to data collection settings, i.e., interview and questionnaire. Second we conducted a meta-analysis of frequency of depression, using the variance-stablizing double-arscine method transformation [9]. Pooled estimates in both the overall (and subgroup) analyses were calculated using the Hartung-Knapp-Sidik-Jonkman method, under the random effect model [10]. Publication bias was examined by Funnel plot and Egger’s regression test. Data from the identical cohorts was reported only once. Where depression was assessed with more than one method in a study, only the results with the most commonly used assessment method were considered. Where data from two or more time points after SAH were available, data from the earlier time point was included in the analysis.
Results
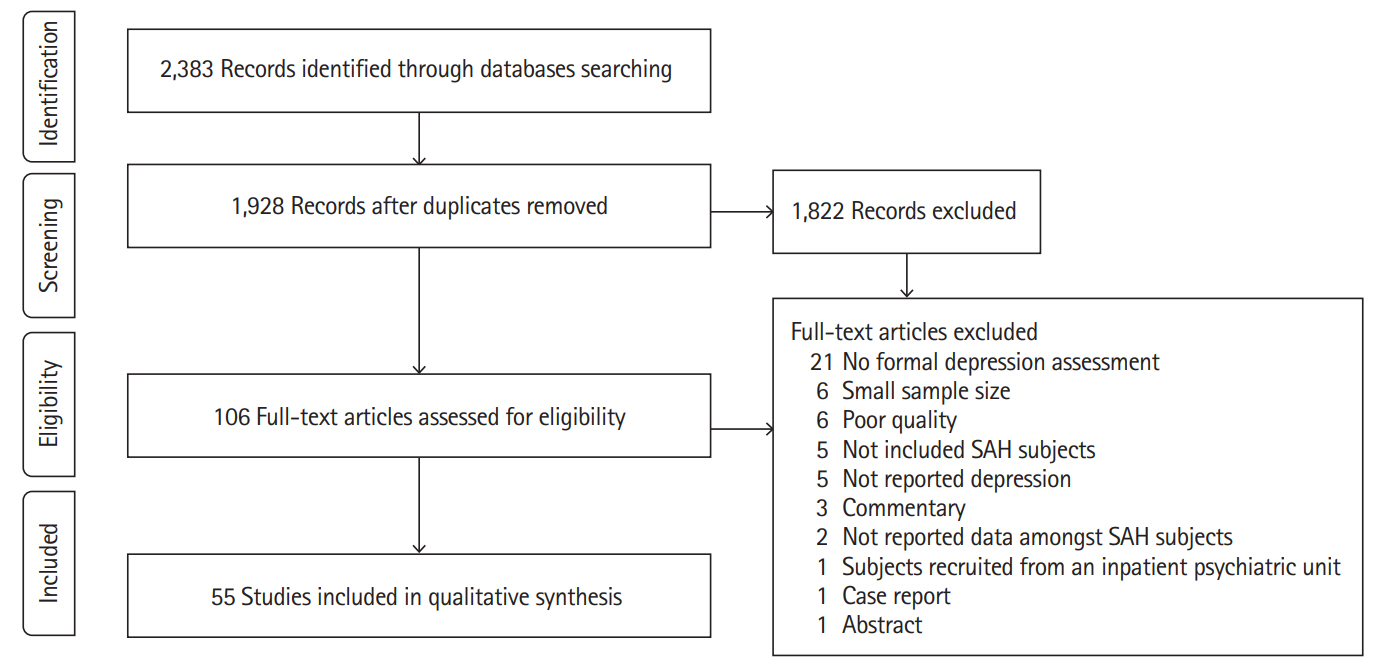
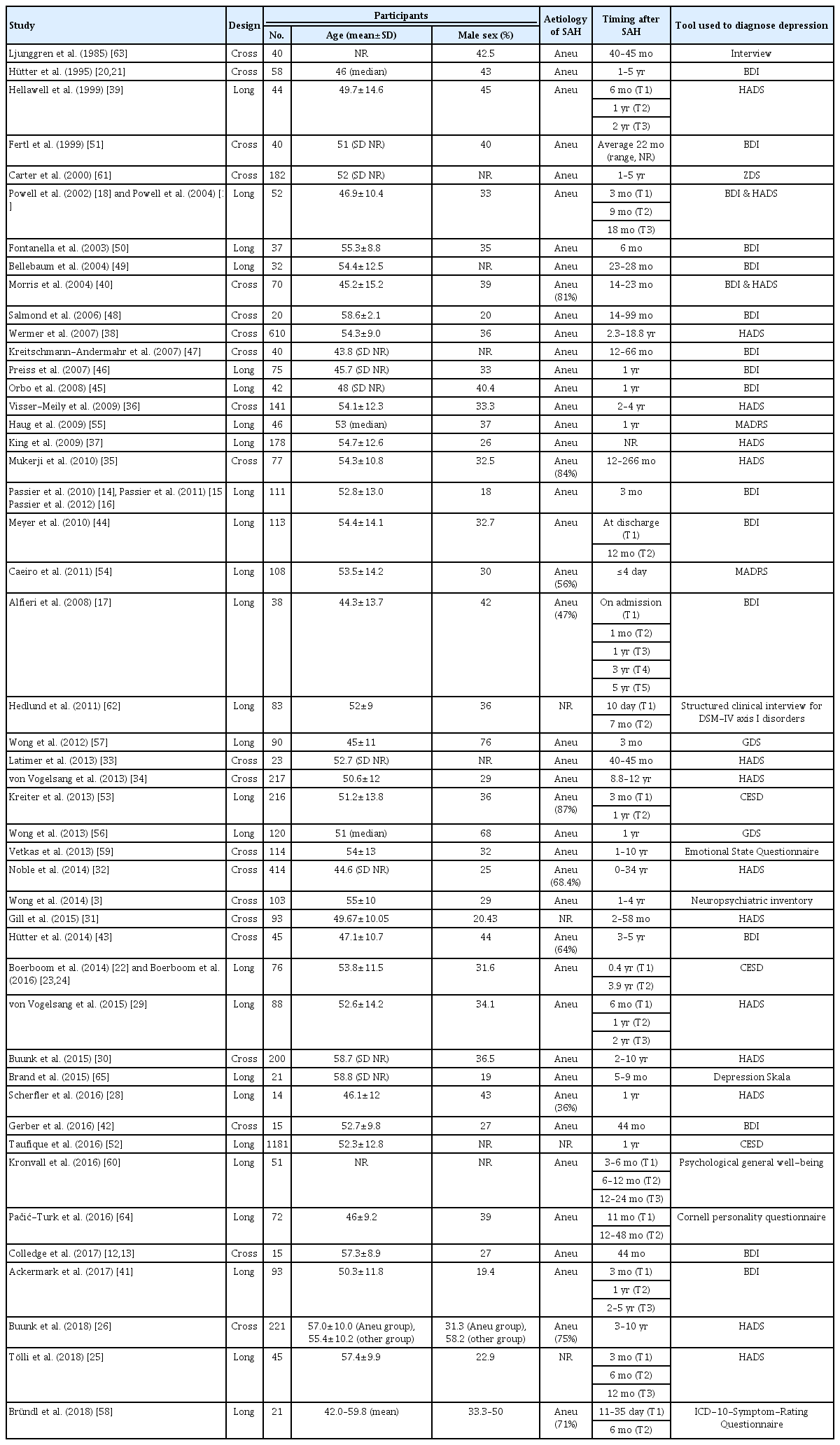
The electronic search identified 2,383 publications potentially eligible for the review. One hundred and six full texts were retrieved for detailed evaluation, of which 51 studies were excluded (Figure 1). Fifty-five studies covering 6,327 patients (range, 40 to 1,181) in 47 cohorts (data from five cohorts were used in 13 publications [12-24]) met the inclusion criteria (Table 1). The majority of studies (46 of 55) included more female than men. Quality of included studies was very different and the STROBE score varied from 14 to 22 (Supplementary Table 1).
Fifty-one of 55 studies (93%) used one of the following screening or rating scales to ascertain the presence of depression: Hospital Anxiety Depression Scale (HADS) [18,19,25-40], Beck Depression Inventory (BDI) [12-21,40-51], Center for Epidemiologic Studies Depression (CESD) [22-24,52,53], Montgomery Åsberg Depression Rating Scale (MADRS) [54,55], Geriatric Depression Scale (GDS) [56,57], International Classification of Diseases 10th Edition (ICD-10)-Symptom-Rating questionnaire [58], Neuropsychiatric Inventory [3], Emotional State Questionnaire [59], Psychological General Well-Being Index [60], and the Zung Depression Scale (ZDS) [61]. Less commonly used measures of depression included the Structural Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV) axis I disorders [62], clinical interview [63], Cornell personality questionnaire [64], and Depression Skala [65] (Table 1).
Twenty-seven studies recruited patients early after SAH and assessed them for depression later. Twenty-six studies recruited patients and collected data at one or more defined time points after SAH (range, 4 days to 10 years) (Table 1).
Frequency, severity, and time course of depression after SAH
Frequency of depression after SAH
Thirty-seven studies (n=5,340) reported the frequency of depression after SAH. The frequency ranged widely depending on the assessment method and how long it was performed after the index SAH.
The frequency of depression reported in the 35 studies (n=5,217) that applied rating instruments/questionnaires was in the range of 0% to 61.7%, with a weighted frequency of 28.1%. Depression was assessed at 4 days to 10.1 years after ictus (Table 2). The weighted frequency of depression at ≤1 and >1 year after ictus were 32.2% and 27.3%, respectively. The frequency in the two studies (n=123) that used interviews to diagnose depression found 20% to 25% (weighted proportion=21.6%) of patients depressed (Table 2) [62,63].
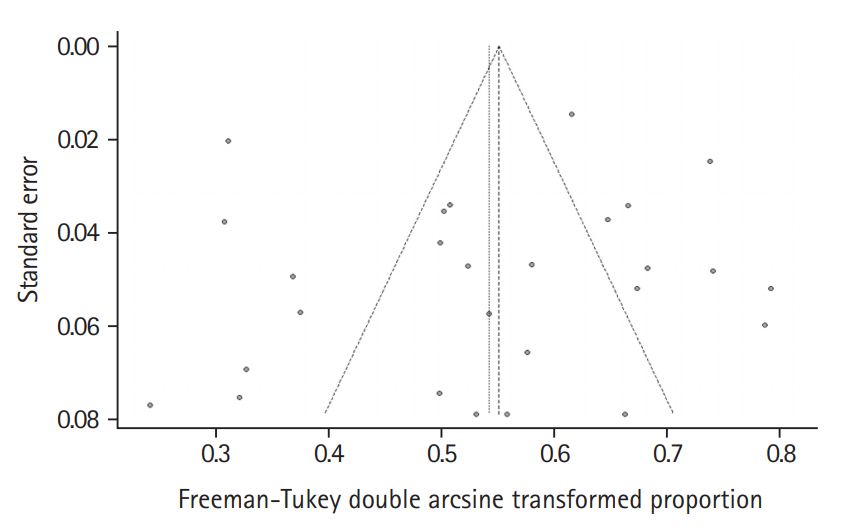
In the meta-analysis, the overall pooled frequency of depression was 26.3% (95% confidence interval [CI], 21.0% to 32.0%). The frequency of depression in the interview and questionnaire studies were 25.0% (95% CI, 12.6% to 39.7%) and 26.3% (95% CI, 20.8% to 32.3%), respectively (Figure 2). The Funnel plot did not suggest any publication bias (P=0.434) (Figure 3).
Severity of depression after SAH
Twelve studies (n=634) assessed the severity of depression using the BDI [14-16,18-21,40,41,44,45,47]. The weighted mean BDI value was 9.9 less than 1 year after SAH and 10.1 1-year or more after; these figures indicate mild to moderate depression [15]. Nine studies (n=1,280) evaluated the severity of depression using the HADS [27-30,33,34,36-38]. The weighted mean HADS value was 5 less than 1 year after SAH and 5.4 1-year or more after; according to a previous validation study [66], both values can be interpreted as an absence of depression [27]. Less commonly used mood scales were the CESD [22-24], MADRS [54,55], GDS [56,57], and one study each used the ZDS [61], Depressions Skala [65], Emotional State Questionnaire [59], Cornell Personality Scale [64], and the Psychological General Well-Being Scale [60] (Tables 1 and 2, Supplementary Table 2).
Time course of depression after SAH
Eight studies (n=539) assessed depression at more than one time point (Supplementary Table 3) [18,19,23,25,29,41,44,64]. In one study (n=113), the proportion of patients with depression increased from 24.8% at discharge to 61.7% at the 12-month follow-up [44]. In another study, 72% of patients with depressive symptoms at 3 months still had symptoms at 2 to 5 years [41]. One study reported an increase in depressive symptoms from 11 months to 12 to 48 months follow-up [64]. On the contrary, five studies (n=261) found no change in depressive symptoms between the first assessment at 3, 6, or 9 months and subsequent follow-up(s) at 6, 9 months, 1, 1.5, 2, or 4 years after SAH [18,19,23,25,29].
In a cross-sectional study, the proportion of patients with depression was not statistically different from patients 2 to 5, 5 to 10, and even more than 10 years after SAH, with 8.3%, 10.7%, and 8.6%, respectively [37]. The weighted frequency of depression was 33% up to a year after SAH and 28% a year or more after [51,53]. There was no relationship between length of follow-up and the severity of depressive symptoms at 39 months after SAH in one study [67].
Association between demographic factors, baseline characteristics of SAH, and depression
Demographic characteristics
Two studies explored the association between age and depression after SAH, all with negative findings [20,40]. One study reported a significant correlation between female sex and depression [54]. The association between sex and depression was not significant in the other three studies (n=314) [34,46]. One study found no association between depression and educational level [65]. Another study reported that non-white ethnicity predicted depression (Table 3) [53].
Premorbid conditions
Three studies (n=411) examined the role of history of depression prior to SAH. Associations were found between depression and previous mood disorders [54]; lifetime history of major depression, or of anxiety or substance use disorder [62], and self-reported history of depression [53]. One study reported that passive coping predicted post-SAH depressive symptoms (Table 3) [41]. Other premorbid conditions were examined in three studies.
One found that depression was associated with lifetime psychiatric comorbidity [62]. The second reported that non-fluency in English and nicotine use predicted depression [53]. The third study observed a borderline association between depression and absence of pre-SAH dementia (Table 3) [54].
Association between clinical features and complications of SAH, comorbidities, biomarkers, and depression
Clinical features and complications of SAH
Two studies (n=128) examined the relationship between neurological outcomes and depression, with negative results (Table 3) [20,40].
Three studies (n=124) compared subtypes of SAH with respect to depression. Depressive symptoms were more common in patients with aneurysmal SAH treated by endovascular treatment or microsurgical clipping than in patients with perimesencephalic SAH [58]. Similarly, aneurysmal SAH patients had a higher mean CESD score and higher rate of depression than their perimensencephalic counterparts in a cohort study [22]. No difference in depressive symptoms between aneurysm versus other bleeding source was observed in a third study (Table 3) [20].
Two studies (n=274) examined the effect of infarction on the frequency of depression following SAH. SAH-related infarction predicted depression [53]. Parietal and/or frontal infarcts were negatively correlated with depression (Table 3) [20].
Four studies (n=167) investigated the effect of SAH treatment. Patients treated with clips had more depressive symptoms than those treated with coils [49]. The other three studies (n=135) reported no difference in depressive symptom scores between patients who were treated by surgical clipping and by endovascular coiling [33,46,50]. One study reported no significant association between measures of depression scores and time from admission until surgery [40]. In a second study, rupture of posterior circulation aneurysms was related to a higher level of depression (Table 3) [34].
Comorbidities
Seven studies (n=399) examined the impact of cognitive function on post-SAH depression. Two studies reported an association between depressive symptoms and objective and self-rated cognitive function and memory function [27,57]. Another study found depression to be more frequent in cognitively impaired patients [51]. In the fourth study, depressive symptoms were significant determinants of cognitive complaints [14]. In contrast, three studies found no association between depressive symptoms and cognitive performance or impairment [25,45,65]. Two studies explored the role of fatigue in post-SAH depression. Both reported a positive correlation between depressive symptoms and fatigue (Table 3) [13,63].
Other comorbid conditions were examined in five studies. Severity of depression was correlated with symptoms of posttraumatic stress disorder in two studies [31,43]. A third study found an association between depressive symptoms and overall physical comorbidity [27]. In the fourth study, disability predicted depressive symptoms [41]. Depression was more frequent in patients with reduced working capacity in the fifth study (Table 3) [51].
Biological markers
Three studies (n=93) assessed the relationship between biological markers and depression following SAH. One reported an association between hair cortisol level and depressive symptoms [13]. Another found that depression was correlated with basal cortisol value, which also predicted depression [47]. The third study found a positive correlation between apolipoprotein E ε4 (APOE-ε4) levels and depressive symptoms (Table 3) [17].
Impact of depression after SAH on patients’ lives
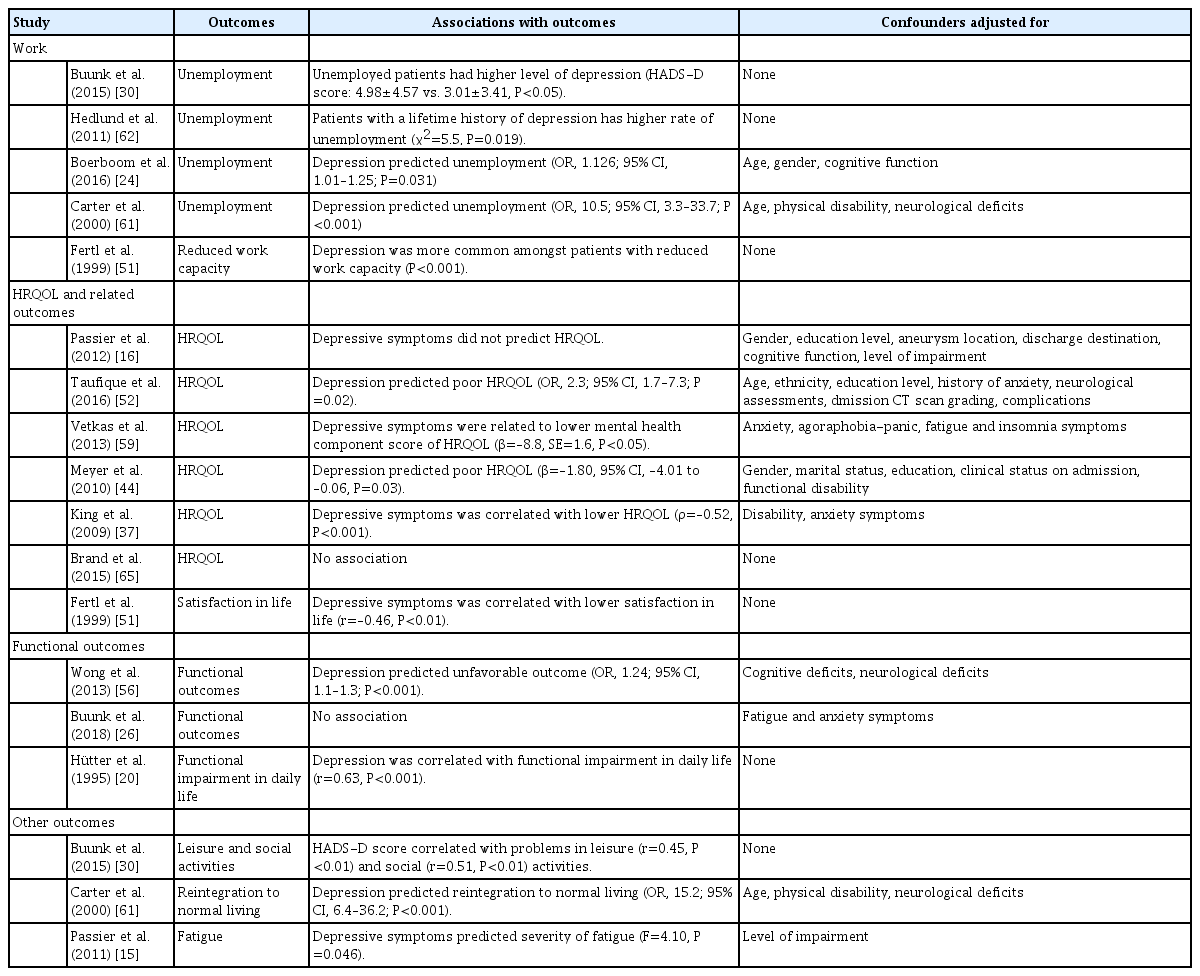
Five studies (n=685) examined the impact of depression on work-related issues. Two studies reported that depression or depressive symptoms were more frequent in patients who were unemployed or with reduced working capacity [30,51]. Lifetime history of major depression and/or post-traumatic stress disorder reduced the likelihood of returning to gainful employment [62]. Two studies concluded that depression or depressive symptoms predicted unemployment (Table 4) [24,61].
Seven studies (n=1,642) looked at health-related quality of life (HRQOL) or life satisfaction. Two studies found a negative association between depressive symptoms and HRQOL or satisfaction with life [16,51]. Four studies found that depression and depressive symptoms predicted poor overall HRQOL or the mental health component of HRQOL [37,44,52,59]. One small-scale study reported no association between depression scores and quality of life (Table 4) [65].
Three studies (n=399) explored how depression influenced functional outcomes. Depression was correlated with self-rated functional impairment in daily life and with the impact of these impairments [20]. Depression predicted poor functional outcomes a year after SAH [56], but this was not confirmed 3 to 10 years after SAH (Table 4) [26].
Other outcomes were assessed in three studies (n=489). Associations were found between depression and problems with leisure and social activities [30], failure to resume previous level of daily life [61], and fatigue (Table 4) [15].
Discussion
To the best of our knowledge, this was the first systematic review of depression after SAH. The weighted frequency of depression following SAH was 28.1%. The severity of depressive symptoms was mild to moderate. Depression after SAH seems to run a chronic course and its frequency does not decrease with time. A host of demographic variables, premorbid and comorbid conditions, as well as clinical features and complications of SAH are related to the risk of depression. Depression has negative impacts on the daily lives of patients with SAH.
The weighted frequency of depression of 28% is similar to the frequency of depression after stroke in general (31%) [68]. The frequency of depression in the included studies varied greatly, probably due to differences in the methods of assessment, patients’ characteristics, and timing of the assessment. The variation in results was more prominent in studies that used questionnaires (0% to 62%) than in those that used interviews to detect depression (20% to 25%) (Tables 2 and 4).
Interviews give a clinical diagnosis whereas questionnaires will assess depressive symptomatology rather than clinical depression. Also, the number of interview studies was considerably smaller.
Longitudinal studies confirmed that depression and depressive symptoms in the later stages of SAH were at least as frequent and severe as in the early stage. Depressive symptoms persisted for long periods after SAH in 72% of patients [41]. Depression after stroke in general is also a chronic condition, with a prevalence and incidence of around 30% and 15% at 1 to 15 years post-stroke [69].
The development of depression after SAH is associated with a variety of factors. The studies included in this review found relationships between depression and female sex, premorbid depression, anxiety, substance use, any psychiatric disorder, and coping styles. Comorbid cognitive impairment, fatigue, post-traumatic stress disorder, and physical disability also increased the risk of depression. The role of the features and complications of SAH in the development of depression was rarely explored, one study suggested that aneurysmal SAH and infarction may be related to depression. The findings on the impact of neurological deficits and treatment modalities for aneurysm repair on depression were inconclusive. Most of the above risk factors have also been found to be related to depression in stroke in general [70]. Further research on psychosocial factors such as pre-stroke life events and the quality of family and social support are warranted [70].
Pituitary dysfunction may occur in up to one in three patients after SAH [71] and could contribute to the development of depression [72]. In support of this theory, an association between low basal cortisol levels and depression has been reported in patients after SAH [47]. One small-scale study reported a possible link between the APOE-ε4 allele and depression [17]. Interestingly, hypercortisolemia, blunted cortisol awakening response [73], and APOE polymorphisms [74] increase the risk of post-stroke depression.
Post-SAH depression was significantly related to functional impairment, unemployment [75] or reduced working capacity, and poor HRQOL. Data on the impact of depression on the costs of hospitalization and mortality related to SAH are lacking [70].
In terms of treatment of post-SAH depression, only one small-scale, open label trial of mindfulness-based psychotherapy has been published. Proper randomized control trials with antidepressants and other treatment modalities are clearly needed. Antidepressants seem to be commonly prescribed in patents with SAH, in a population-based cohort of 940 patients with SAH, 27% had continuous antidepressant use [76]. On the other hand, the use of selective serotonin reuptake inhibitors, a commonly prescribed antidepressant, in general population was associated with increased risk of intracranial hemorrhage, particularly in the first 30 days of use and when used currently with anticoagulants [77]. Antidepressants are effective in the treatment [78] and prevention of post-stroke depression [79], and non-pharmacological treatment modalities including ecosystem-focused therapy, life review therapy, problem solving therapy, meridian acupressure, transcranial magnetic stimulation, music therapy, exercise, light therapy, motivational interviewing, and robotic-assisted neurorehabilitation could also be trialed [80].
This systematic review has several methodological strengths. An extensive search strategy was used so it is unlikely that relevant studies were missed. Two authors extracted pre-specified data independently, thus reducing the chance that any errors in data extraction would have gone undetected. A major limitation is the inclusion only of studies published in English.
There were several methodological shortcomings in the included studies that weakened the robustness of the review. First, the study design was heterogeneous, including longitudinal [27], cross-sectional [26], or retrospective [32] single site cohorts. Second, while most studies recruited hospitalized [26] subjects, some employed clinic [33,38,42], population [60], or support group [30,31] based sampling. Third, a number of studies involved bias sample, such as particular location [32,54,81], investigation [3] or treatment received [11,37,45], or neurological outcome [65,82] of SAH. Fourth, many studies had small sample size. Fifth, the timing of mood assessment various varied from acute [53,55] to chronic stage [33] of SAH recovery. In addition, most of them assessed depression only once; in cross-sectional studies, subjects were assessed at different time point following SAH. Sixth, most studies used scales to measure depression, whereas some employed, a single question [83], or clinical interview [61,62]. Seventh, some authors described the baseline characteristics of SAH but did not relate them to the presence or severity of depression. Eighth, the majority of studies did not measure and adjust for potential confounders, such as personality, level of social support, recent life events, or previous depression with multivariate analysis. Future studies should consider prospective multi-center design, careful and non-selective sampling method, large sample size, assessment of depression at multiple time points with structural psychiatric interview, and detailed measurement and analysis of demographic and clinical characteristics and other possible confounding factors.
Implication for clinicians
Clinicians involved in the long-term care of SAH survivors need to be aware that post-SAH depression is common, runs a chronic course, and has a negative impact on patients’ lives. Clinicians should routinely ask about depression when they review SAH patients and they should refer patients with suspected depression for psychological and/or psychiatric evaluation and treatment.
Directions for future research
More longitudinal studies are needed to determine the time course of depression after SAH using standardized diagnostic interviews. More studies assessing the relationship between depression and demographics, premorbid and comorbid conditions and complications of SAH are required to elucidate the relationship between depression and the consequences of SAH. Research on the association between depression and pituitary dysfunction, changes in neurotransmitter metabolism, and disruption of brain circuits after SAH is also warranted to clarify the pathogenesis of post-SAH depression. Finally, randomized controlled clinical trials on potential treatments for SAH-related depression are also needed.
Conclusions
Depression is common after SAH and seems to persist. The development of depression after SAH is associated with a variety of factors. Post-SAH depression had negative impacts on patients’ daily life. Further research is needed to clarify its time course and identify the neuroendocrine and neurochemical factors and brain circuits associated with the development of post-SAH depression. Randomized controlled treatment trials targeting SAH-related depression are warranted.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2019.02103.
Quality assessment of studies
Severity of depression after subarachnoid hemorrhage
Time course of depression after subarachnoid hemorrhage
Notes
Disclosure
The authors have no financial conflicts of interest.