Introduction
Over the years, number of patients with end-stage renal disease (ESRD) have been steadily increasing. Even though, the mortality rates have decreased, it still remains high (165 per 1,000 patient-years) [1]. Cardiovascular (CV) causes accounts for 48% of all deaths among ESRD patients. In particular, there is an increased risk of atrial fibrillation (AF) and thromboembolism such as deep vein thrombosis, pulmonary embolism, transient ischemic attack (TIA) and cerebrovascular accident (CVA) [2]. In a study of 40 hemodialysis (HD) patients, onset of AF was noted to be more often on the day of HD and specifically during the HD treatment, suggesting HD as a trigger for AF in itself [3]. Larger left atrium, higher systolic blood pressure, and increased age were additional risk factors for AF [3,4]. In addition to AF, risk of stroke (event rate of 5.61 per 100 person years) in ESRD patients is greatly increased as compared to those without renal impairment (event rate of 3.61 per 100 person years) [5], which is very concerning due to the already increased CV mortality among ESRD patients.
Systemic anticoagulation is routinely recommended for CVA/TIA risk reduction in patients with AF, with CHA2DS2-VASc risk factors. But, the case is not the same with patients with the kidney disease, as there have been concerns of increased risk of bleeding with the use of systemic anticoagulants in the ESRD population [5]. As noted in the United States Renal Data System (USRDS) database, in 2016, only 32.5% of HD, 31.5% of peritoneal dialysis (PD) and 32.6% of transplants patients with AF were prescribed warfarin and 9.4% of HD, 9.4% of PD and 17.8% of transplant patients were given direct oral anticoagulant (DOACs) [2]. As per the Kidney Disease/Improving Global Outcome (KDIGO), there is a lack of high quality evidence to recommend warfarin or single or dual antiplatelet agents for stroke prevention in ESRD with AF and to reduce bleeding risk, low dose apixaban (2.5 mg orally twice a day) or rivaroxaban (15 mg once a day) may be considered. However, a team based and multi-disciplinary approach with annual re-evaluation of treatment goals and risk-benefit assessment was recommended [6].
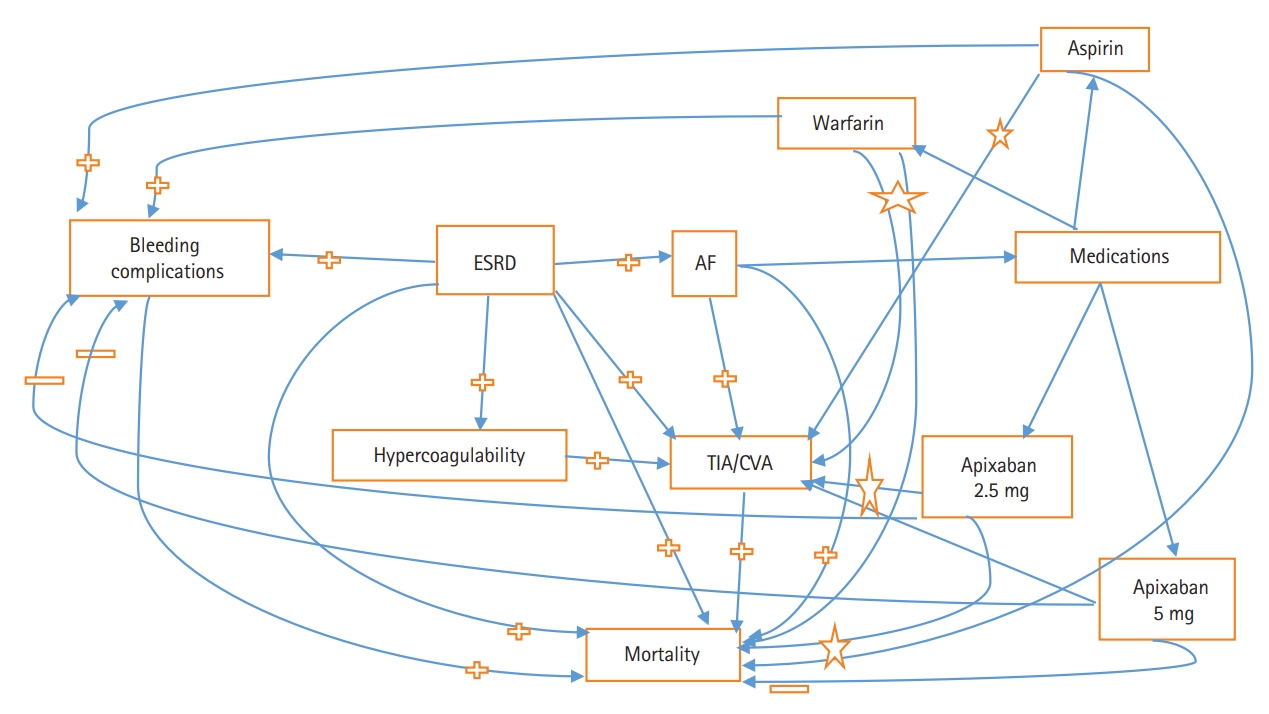
Hence, widespread sense of confusion is prevailing in the medical community regarding systemic anticoagulation in ESRD patients with AF (Figure 1). In a recent multinational survey of cardiologist and nephrologists, substantial intra and inter specialty heterogeneity was noted in terms of use, dosing and monitoring of oral anticoagulant in patients with the chronic kidney disease (CKD) [7]. In this review, we would like to discuss the current evidence and pros/cons of systemic anticoagulation in a particularly vulnerable patient population with the condition of ESRD.
Epidemiology of AF and CVA in ESRD
As per the USRDS, prevalence of AF in HD, PD and transplant patients were reported as 19.6%, 14.1%, and 10.9%, respectively. Prevalence of AF in HD patients showed considerable differences among different age groups as 5.5% in age 22 to 44 years, which dramatically increased to 24.6% in 65 to 74 years and 33.2% in >74 years of age group. Similar patterns were observed in PD and transplant patients as well [2]. In the most recent analysis of 117,023 dialysis patients from Taiwan, patients on HD and PD had an incidence of AF as 8.8 and 7.8 events per 1,000 person-years, respectively [8]. AF is a known risk factor for stroke. In a Canadian cohort of 5,502 HD patients with AF, authors found incidence rate of stroke as 26.7 per 1,000 patient-years [9]. Race and ethnicity have also been shown to have an impact on risk of CVA in the presence of ESRD and AF. In a cohort of 56,587 ESRD patients on HD; Blacks (hazard ratio [HR], 1.13; 95% confidence interval [CI], 1.02 to 1.24), Hispanics (HR, 1.15; 95% CI, 1.01 to 1.30), and Asians (HR, 1.16; 95% CI, 1.02 to 1.35) were all at the increased risk of stroke as compared to whites [10]. Risk of ischemic stroke is much higher than the risk of hemorrhagic stroke in ESRD patients (21.1 per 1,000 patient years vs. 4.7 per 1,000 patient years) [11]. PD patients are found to be at lower risk of hemorrhagic stroke as compared to HD patients (HR, 0.75; 95% CI, 0.58 to 0.96) and no such significant difference was noted for ischemic stroke. However, regardless of dialysis modality, when compared to the general population, the risk of hemorrhagic stroke among HD (HR, 6.83; 95% CI, 5.89 to 7.92) and PD (HR, 6.15; 95% CI, 4.83 to 7.84) patients is increased far more than the risk of ischemic stroke among HD (HR, 2.88; 95% CI, 2.6 to 3.19) and PD (HR, 3.21; 95% CI, 2.69 to 3.83) patients [12]. Thirty days mortality risk with hemorrhagic stroke as compared to ischemic stroke is also significantly higher (53.4% vs. 17.9%) [11].
Occurrence of AF across the various spectrum of kidney diseases portend poor clinical outcomes. AF in transplant recipients is also associated with graft loss and mortality. But the risk of CVA/TIA is significantly lowered with kidney transplantation (24.6 events per 1,000 patient years) as compared to those on dialysis (45.6 events per 1,000 patient years) [13]. Incident AF in patients with CKD is also associated with an increased risk of developing ESRD, stroke, and death [14,15]. In a retrospective analysis of incident CKD patients (n=16,451) from 2000 to 2013, authors reported 612 patients with AF at baseline (prevalent) and 588 patients who developed AF (incident) during the course. Both incident and prevalent AF group had higher risk of developing ESRD, stroke or systemic thromboembolism and death with highest risk among CKD patients with incident AF (HR, 2.90; 95% CI, 2.73 to 3.08; P<0.0001 for ESRD) (HR, 1.82; 95% CI, 1.67 to 1.97; P<0.0001 for stroke) (HR, 2.49; 95% CI, 2.36 to 2.62; P<0.0001 for all-cause mortality) [16]. Incident ESRD patients with baseline AF were reported to have higher CV and cerebrovascular disease (HR, 1.87; 95% CI, 1.26 to 2.78; P<0.01) including death (HR, 1.87; 95% CI, 1.17 to 3.0; P<0.01) as compared to those without AF [17]. Incident AF in prevalent ESRD patients was also noted to be associated with increased mortality risk [4]. Therefore, AF in the presence of kidney disease deserves special attention and management.
Pathophysiology of AF and CVA in ESRD
Pathophysiology behind increased risk of AF among ESRD is the increased prevalence of traditional risk factors for AF. In addition, ESRD state is also associated with chronic fluid and pressure overload, activation of sympathetic nervous system and renin angiotensin aldosterone system, inflammation, oxidative stress, electrolytes abnormalities, hemodynamic changes, extracellular remodeling, and ventricular hypertrophy which lead to atrial electrophysiological and structural changes and eventually to AF [18]. Presence of congestive heart failure (CHF) is increased as 40.4%, 28.3%, and 14.4% in HD, PD, and transplant patients, respectively. AF in the presence of increased age, hypertension, and CHF is known to be associated with an increased risk of CVA and TIA. Without any surprise, HD, PD, and transplant patients are all reported to have an increased prevalence of CVA/TIA events as 16.3%, 12.4%, and 7.1%, respectively [2]. Cerebral microbleed (CMB), focal deposits of hemosiderin, is another pathophysiological aspect, which is associated with an increased risk of intracerebral hemorrhage (ICH), ischemic and hemorrhagic stroke, and is quite common among HD patients (54%) [19]. Mechanism for CMB includes uremia induced alteration in actin cytoskeleton and claudin-5 expression, a tight junction protein in endothelial cells [20]. Impaired renal function, age, and hypertension were all found to be associated with CMB in patients with ischemic stroke [21]. In a meta-analysis of studies involving recent ischemic stroke, documented AF and long term anti-coagulation therapy, future odds of ICH were noted to be associated with the presence of CMB at baseline (odds ratio [OR], 2.68; 95% CI, 1.19 to 6.01; P=0.017) and the number of CMB (OR for ICH, 5.5; 95% CI, 2.07 to 14.66; P=0.001 with CMB ≥5). In another review of 504 patients with ischemic stroke and AF, presence of CMB ≥5 was associated with all-cause (HR, 1.99) and ischemic stroke mortality (HR, 3.39). Moreover, hemorrhagic stroke mortality (HR, 5.91) was particularly increased with lobar CMB [22]. Hence, detailed information on CMB can further help risk-stratify ESRD patients [23].
In view of above mentioned epidemiology, it would seem apparent that ESRD patients with AF and such increased risk of CVA/TIA and mortality be considered for systemic anticoagulation with minimal side effects and aim to reduce the morbidity and mortality related with thromboembolic events. However, it is important for the clinicians to be aware about their hypercoagulable state and concomitant increased risk of bleeding.
Pathophysiology of hypercoagulable state in ESRD
Risk of deep vein thrombosis is significantly higher among ESRD patients as compared to non-ESRD patients (adjusted HR [aHR], 13.92; 95% CI, 9.25 to 20.95), likely due to their hypercoagulable state [24]. Patients with kidney disease have an increased level and/or activity of numerous pro-inflammatory and pro-coagulant factors such as C-reactive protein, cystatin C, interleukin 6 (IL-6), fibrinogen, factor VII, VIII, IX-XII, IL-6, tumor necrosis factor α soluble receptor-1, intracellular adhesion molecule-1, vonWillebrand factor, plasminogen activator inhibitor-1, homocysteine, thromboplastin, and fibrinopeptide A. In addition, there is a decreased level of anticoagulant protein C [24].
Amongst dialysis patients, clot lysis time has been noted to be significantly prolonged, suggesting a hypofibrinolytic state [25]. As compared to HD, patients on PD may be at higher risk of thrombosis due to increased pro-coagulant factors and macrophage activation, increased presence of thromboplastin and plasminogen activator in the peritoneal cavity from exposure to the dialysate [26]. Kidney transplant recipients also exhibit pro-thrombotic and inflammatory state. These patients have increased level of fibrinogen, d-dimer, IL-6, thrombin generation, pro-thrombin activation fragment, post-transplant erythrocytosis leading to hypercoagulable state [27].
Pathophysiology of increased risk of bleeding in ESRD
On one hand ESRD patients are hypercoagulable, while on the other, they are at an increased risk of bleeding due to abnormal primary hemostasis involving vasoconstriction, platelet adhesion, and aggregation rather than impairment of secondary hemostasis mechanisms (coagulation pathways) or fibrinolysis [28]. There is a great degree of platelet dysfunction among ESRD owing to decreased platelet activity, recruitment, adhesion, and aggregation along with impaired interaction among platelets and endothelium [29,30]. In a study evaluating hemostasis among ESRD patients, authors found decreased thrombin generating capacity and clopidogrel like platelet dysfunction [25,31]. Furthermore, patients on HD are frequently given intravenous heparin during the treatment which can increase the risk of bleeding. Mechanisms behind platelet dysfunction and impaired interaction between platelet and endothelium in ESRD patients are listed in Table 1.
Pros and cons of AF and systemic anticoagulation in ESRD
ESRD patients with AF, without treatment with systemic anticoagulation or antiplatelet agents, have an increased risk of ischemic stroke (incidence of 6.9 per 100 patient years) [32]. In a study of 5,629 incident ESRD patients with AF, rate of ischemic and hemorrhagic stroke were 22.8 and 5.0 per 1,000 patient years, respectively. However, chronic AF was noted to be associated with increased risk of ischemic stroke only (HR, 1.26; 95% CI, 1.06 to 1.49; P=0.0005), not hemorrhagic [33]. Chang et al. [8], found comparable incidence of ischemic stroke in HD and PD patients (HR, 1.07; 95% CI, 0.80 to 1.44) but much lower risk of hemorrhagic stroke in PD patients as compared to HD patients (HR, 0.34; 95% CI, 0.13 to 0.90). Kidney transplant patients are also at increased risk of AF and CVA/TIA which occurred in over 7% of recipients and within 3 years of transplantation. Incidence of AF is reported to be highest in peri-transplant period [13].
Occurrence of stroke among ESRD patients leads to poor outcomes. In an analysis of matched cohort of patients hospitalized with acute ischemic stroke, risk of in-hospital mortality (7.6% vs. 5.2%, P<0.001), sepsis (4% vs. 2.5%, P<0.001), median length of stay (6 days vs. 5 days, P<0.001), and median cost of care (11,046$ vs. 10,357$, P<0.001) were all increased among ESRD patients as compared to the non-dialysis patients. Moreover, 52.6% of ESRD patients admitted with stroke were not able to be discharged home. Utilization of gastrostomy (5.6%) and mechanical ventilation (2.8%) was also increased owing to stroke related complications [34].
ESRD patients especially on HD are at an increased risk of bleeding especially upper gastrointestinal (GI) bleeding secondary to angiodysplasia and erosive esophagitis [35]. It can also be due to other causes such as, arteriovenous access related, oral/nasal or genitourinary bleeding [35]. Risks of in-hospital mortality, need for blood transfusion, re-bleeding, hospitalization, and length of stay are all increased among ESRD patients with GI bleeding [36]. Risk of both non-traumatic and traumatic subdural hemorrhage is increased among ESRD patients which is much more pronounced with HD modality as compared to PD (aHR, 1.62; 95% CI, 1.17 to 2.33). History of AF and use of medications such as anti-platelets and warfarin were associated with an increased risk of subdural hemorrhage in this cohort. Subdural hemorrhage is also associated with an increased risk of mortality at 30 days [37]. Risk of bleeding is significantly increased in patients with kidney transplantation too, as compared to general population (relative risk, 8.2; 95% CI, 6.9 to 9.7) [38]. In a study of 255 ESRD patients, risk of major bleed was increased greatest with use of aspirin and warfarin (6.3% per person-year of exposure; 95% CI, 2.1 to 14.8; P=0.006). Such risk was 3.1% per person of year exposure to warfarin alone (95% CI, 1 to 7.3; P=0.07) and 4.4% per person of year exposure to aspirin alone (95% CI, 2.3 to 7.7; P=0.002) [39]. However, there was no increased risk of intracranial hemorrhage or GI bleeding or death with use of warfarin. These findings raise concerns regarding increased risk of stroke without anticoagulants but increased risk of bleeding when these medications are given and therefore, confusion about therapeutic choices prevails.
Predicting stroke versus bleeding
We need tools to stratify ESRD patients regarding their risk of CVA/TIA and bleeding to better identify patients who could be offered anticoagulants safely. At present, CHA2DS2-VASc (CHF, hypertension, age ≥75 years, diabetes mellitus, stroke/TIA, vascular disease, age 65 to 74 years, and female sex) is the recommended model for stroke risk stratification in patients with AF [40]. Even though, presence of CKD and ESRD is associated with an increased risk of TIA/stroke in patients with AF, it is not included in this risk scoring. Observational studies have evaluated the performance of these risk scoring methods. In an ESRD cohort of 10,999 patients, both CHADS2 and CHA2DS2-VASc scores were significant predictors of stroke, but CHA2DS2-VASc score performed better than the CHADS2 score [32]. Anticoagulant and risk factors in AF (ATRIA), another scoring system proposed which also gave points to proteinuria and estimated glomerular filtration rate <45 mL/min/1.73 m2 or ESRD, has performed better in term of identifying low stroke risk patients [40]. Lately, The Global Anticoagulant Registry in the FIELD-Atrial Fibrillation (GARFIELD-AF) score which also included CKD stage 3 and 4 has been shown to be superior to CHA2DS2-VASc in predicting risk of stroke and mortality [41].
There are various risk scoring systems developed to assess bleeding risk as well. While comparing Hypertension, Abnormal liver/kidney function, Stroke, Bleeding, Labile INR, Elderly, Drugs or alcohol (HAS-BLED), Outcomes Registry for Better Informed Treatment (ORBIT), Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA), and Hepatic or Renal Disease, Ethanol Abuse, Malignancy, Older Age, Reduced Platelet Count or Function, Re-Bleeding, Hypertension, Anemia, Genetic Factors, Excessive Fall Risk and Stroke (HEMORR2HAGES) bleeding risk scores, Proietti et al. [42], found best discrimination and calibration with ORBIT score. GARFIELD-AF score which performs comparable to HAS-BLED risk score, also provides comparison of different anticoagulant choices. But, most of these scoring systems do not take ESRD status into consideration. Hence, ESRD patients are greatly in need of a separate risk stratification model taking into account their predisposing factors for stroke/bleeding, co-morbidities, CMBs, modality and duration of dialysis so that decision making can be guided regarding treatment options to reduce the risk of TIA/stroke and bleeding as well. At present, antiplatelet agents, warfarin and DOAC are the medications available for use in patients with AF for CVA/TIA prevention, which will be discussed below in the context of ESRD patients.
Use of antiplatelet agents in ESRD for CVA/TIA prevention
A recent study focused on effectiveness and safety of antiplatelet agent in ESRD patients with ischemic stroke. This study analyzed 1,936 ESRD patients with first ischemic stroke, primary outcome being death and readmission for stroke and secondary outcome as bleeding, stroke, acute myocardial infarction, and death. Authors found decreased risk for the primary outcome with aspirin treatment (HR, 0.671; P<0.001). Specifically, risk for readmission with stroke (HR, 0.72; P=0.002) was significantly reduced with aspirin without an increased risk of bleeding (HR, 0.885; P=0.291) [43]. However, in another study of 901 AF patients requiring renal replacement therapy (RRT), use of aspirin or aspirin with warfarin was not associated with the decreased risk of stroke or thromboembolism [5]. Choice of RRT such as HD, PD, or transplantation did not affect the outcome in these patients either [5]. In a retrospective Danish study, risk for the composite of stroke and bleeding with use of aspirin in low stroke risk (CHA2DS2-VASc score=0) patients on HD (HR, 1.17; 95% CI, 0.61 to 3.60) was similar to those on PD (HR, 1.83; 95% CI, 0.51 to 6.49) with P-value of 0.749 for PD and HD interaction. However, in patients at high risk for CVA (CHA2DS2-VASc score ≥2), risk for such outcomes were lower in PD patients (HR, 0.74; 95% CI, 0.42 to 1.31) as compared to HD patients (HR, 1.07; 95% CI, 0.80 to 1.42) with significant P-value (0.041) for PD and HD interaction [44].
On the contrary, antiplatelet medication use in ESRD was associated with an increased risk of bleeding especially among those with diabetes mellitus [45]. Olesen et al. [5], also reported increased risk of bleeding in ESRD with aspirin use (HR, 1.63; 95% CI, 1.18 to 2.26; P=0.003). Risk of bleeding was slightly more pronounced with combination of warfarin and aspirin (HR, 1.71; 95% CI, 0.98 to 2.99; P=0.06). Bleeding risk (ICH) was more pronounced with aspirin use among patients with severe hypertension (pre-dialysis systolic blood pressure >160 mm Hg) [46]. Due to conflicting data regarding risks and benefits of antiplatelet medication use among ESRD patients with AF, there is no consensus or guideline regarding their routine use. Results from a randomized controlled trial to assess aspirin use in ESRD patients to reduce the risk of thrombotic events are awaiting [47].
Use of warfarin in ESRD for CVA/TIA prevention
Role of warfarin, a vitamin K antagonist, remains unclear and uncertain in ESRD with AF for CVA/TIA prevention. In a retrospective analysis of 56,587 HD patients with AF, warfarin use was associated with lower risk of ischemic stroke and death without an increased risk of GI bleeding [10]. In a meta-analysis of all observational studies, authors did not find a statistically significant reduction in stroke risk (14 studies; 20,398 participants; aHR, 0.77; 95% CI, 0.55 to 1.07) [48]. Surprisingly, there was no statistically significant increased risk of intracranial hemorrhage (four studies; 15,726 participants; aHR, 1.93; 95% CI, 0.93 to 4.00) or GI bleeding (three studies; 14,693 participants; aHR, 1.19; 95% CI, 0.8 to 1.76) or death (seven studies; 16,172 participants; aHR, 0.89; 95% CI, 0.72 to 1.11) with use of warfarin either. On the contrary, Abuhasira et al. [4], had reported a higher risk of CVA with use of warfarin in ESRD patients as compared to no warfarin (24.2% vs. 12.9%, P=0.026) and statistically non-significant increased risk of major bleeding (16.7% vs. 9.2%, P=0.09). In particular, risks of hemorrhagic stroke (aHR, 1.22; 95% CI, 1.03 to 1.46) and calciphylaxis (aHR, 1.49; 95% CI, 1.05 to 1.91) were increased with warfarin use [10]. In another meta-analysis of 56,146 patients with ESRD and AF, warfarin use was again not associated with reduction in stroke risk (HR,0.92; 95% CI, 0.74 to 1.16). Instead, it was associated with increased risk of bleeding (HR,1.21; 95% CI, 1.01 to 1.44) [49]. Similar results were reported in meta-analysis of 17,380 HD patients whereby risk reduction of ischemic stroke (HR, 0.74; 95% CI, 0.51 to 1.06) and mortality (HR, 1.00; 95% CI, 0.92 to 1.09) was not significant with warfarin use. Rather, a 21% increase in total bleeding risk (HR, 1.21; 95% CI, 1.03 to 1.43) was noted [50]. There are no specific guidelines for or against use of warfarin in kidney transplant recipients. In a transplant cohort, warfarin use did not have impact on risk of ischemic or hemorrhagic stroke or GI bleeding [51]. There is a paucity of data regarding warfarin use in PD patients and AF. In a subgroup analysis, PD patients with a CHA2DS2-VASc score=0 were at quite high risk of stroke/bleeding (HR, 4.67; 95% CI, 1.56 to 14.03) and all cause death (HR, 2.61; 95% CI, 1.29 to 5.28) with the use of warfarin. This was noted not to be significant for stroke/bleeding (HR, 1.19; 95% CI, 0.63 to 2.24) or all cause death (HR, 0.78; 95% CI, 0.56 to 1.11) for those with CHA2DS2-VASc score ≥2 [44]. These findings are worrisome regarding safety and efficacy of warfarin in dialysis patients with AF, who are already at an increased risk of bleeding and hemorrhagic strokes.
Use of DOACs in ESRD for CVA/TIA prevention
DOACs are the preferred choice of anticoagulants in the general population owing to reduced risk of bleeding, and non-inferior and/or superior risk reduction in stroke, as compared to warfarin. In addition, there is no need for therapeutic monitoring or dietary restrictions with the use of DOACs which is a desired feature for any medication use in ESRD [52]. Unfortunately, randomized trials involving these agents did not include ESRD patients. Hence, data regarding safety and efficacy of these agents is solely based upon observational data. Direct thrombin inhibitors such as dabigatran are not approved for use in patients with ESRD [52]. Similarly, other novel oral anticoagulants, which inhibit factor Xa, such as edoxaban and rivaroxaban are not approved for use in ESRD. In a single dose study of rivaroxaban 15 mg given 3 hours after or 2 hours before a 4 hours HD session, systemic exposure was 56% and 47% higher, respectively, as compared to patients with normal kidney function [53]. While rivaroxaban is not approved for use in ESRD with AF for stroke risk reduction at present, it may change in future with more study results and data analysis.
Recently in 2014, apixaban which is also a factor Xa inhibitor, was approved for use in ESRD, even though trials involving apixaban did not include ESRD patients [54,55]. Dosage recommendations from manufacturers are solely based upon one study whereby single dose exposure of apixaban 5 mg to ESRD patients resulted in 36% higher area under curve (AUC) and no increase in maximum concentration (Cmax) of drug as compared to healthy subjects. HD session led to 13% and 14% reduction of Cmax and AUC for apixaban, respectively [56]. However, in a subsequent pharmacological study of apixaban in HD patients, dosage of 5 mg twice daily (standard dose) resulted in supra-therapeutic levels and were recommended to be avoided. However, drug exposure from dosage of 2.5 mg twice daily (reduced dose) in HD patients was comparable to dosage of 5 mg twice daily in patients with preserved renal function [55]. Hence, there is great degree of confusion about its dosage for ESRD patients which is further compounded by conflicting observational data.
Among ESRD patients treated with dosage of apixaban 2.5 mg twice daily (n=1,317) and apixaban 5 mg twice daily (n=1,034), authors did not found any increased risk of bleeding with standard dose as compared with reduced dose. However, the risks of stroke (HR, 0.64; 95% CI, 0.42 to 0.97; P=0.04) and death (HR, 0.63; 95% CI, 0.46 to 0.85; P=0.003) were lower with standard dose [57]. In a meta-analysis involving five studies and 43,850 advanced CKD and ESRD patients, apixaban was associated with lower risk of bleeding but similar risk of thromboembolic event when compared to warfarin treatment [58]. In a recent analysis of 16 studies involving 71,877 ESRD patients with AF, authors did not find a decreased risk of CVA/TIA with use of apixaban or warfarin. Mortality risk was much lower with use of apixaban 5 mg when compared to warfarin (HR, 0.65; 95% CI, 0.45 to 0.93), apixaban 2.5 mg (HR, 0.62; 95% CI, 0.42 to 0.90), and no anticoagulants (HR, 0.61; 95% CI, 0.41 to 0.90) [59]. On the other hand, in a retrospective study of 114 ESRD patients on HD, total bleeding events were increased with continuation of apixaban use (OR, 13.07; P=0.018), increased total daily dose (OR, 1.72; P=0.003), and total dialysis sessions while on apixaban (OR, 2.04; P=0.033) [60]. Use of other DOACs such as dabigatran and rivaroxaban is also associated with significant risk of bleeding as compared to apixaban [59].
Randomized controlled trials involving apixaban and warfarin in ESRD/AF are underway (NCT02933697 and NCT02942407) which hopefully will shed more light on appropriate dosage, safety and efficacy of these agents in ESRD. As noted, most of available data pertains to HD patients. There is paucity of dosing, safety and efficacy information on use of DOACs in patients treated with PD and kidney transplantation. In a subgroup analysis, risk of stroke/systemic embolism with apixaban as compared to warfarin treatment was not impacted by choice of modality as HD (HR, 0.85; 95% CI, 0.66 to 1.09) or PD (HR, 1.26; 95% CI, 0.57 to 2.82; P for interaction between HD and PD=0.36). Also, death risk due to choice of apixaban versus warfarin was not impacted by whether patient on HD (HR, 0.83; 95% CI, 0.70 to 1.00) or PD (HR, 0.98; 95% CI, 0.52 to 1.83; P for interaction between HD and PD=0.62). Similar trends were noticed for major bleeding and GI bleeding [57]. Table 2 summarizes the major studies investigating role of antiplatelet agent as aspirin, vitamin K antagonist as warfarin and DOAC as apixaban in ESRD patients with AF [4,5,10,43,44,57-59,61-65].
Conclusions
ESRD is a complex scenario whereby usual interventions do not often provide desired results as one would observe in the general population. There is a great degree of paradox in how ESRD patients respond to a clinical condition and treatment given. AF is one such unique co-morbidity which greatly increases the risk of CVA/TIA and mortality. Unfortunately, therapy with antiplatelet agents and systemic anticoagulants do not consistently result in the improved outcomes, instead may add further to the risk of complications. There are concerns, confusion and consequences of each step we take or hold back especially in ESRD patients with AF regarding the anticoagulation question. Due to the contradictory results and outcomes, it is not possible to argue for or against the use of any given therapeutic agent in particular. Hopefully, data from randomized controlled trials in near future will help us to overcome these challenges and guide us in making well informed clinical decisions.





 ; Decreased,
; Decreased,  ; Inconclusive, ☆.
; Inconclusive, ☆.