Clinical Outcomes of Mechanical Thrombectomy in Stroke Tandem Lesions According to Intracranial Occlusion Location
Article information
Dear Sir:
Tandem lesions (TLs), depict around 20% of acute ischemic stroke (AIS) in patients with large vessel occlusion (LVO) [1]. TL are poorly represented in thrombectomy clinical trials [2], leading to uncertainty in the homogenization of the therapeutic strategy [3]. Clinical benefit of mechanical thrombectomy (MT) in LVO alone is clear [2] and untreated AIS due to tandem occlusions generally has a poor prognosis [4]. There is growing evidence about efficacy and safety of MT in TL [5,6]. However, the evidence of intracranial lesion impact on endovascular treatment in TL is still lacking.
We extracted the information related to the MT performed in our tertiary hospital from a local registry carried out prospectively between January 2017 and January 2019. Patients were eligible for study if they had anterior AIS within 6 hours of symptom onset or eligibility on wake-up stroke and 6 to 24 hours, confirmed by advanced brain imaging (computed tomography [CT] perfusion) [1]. Eligibility criteria were (1) 18 years of age and older; (2) pre-stroke modified Rankin Scale (mRS) 0–2; (3) vascular occlusion located in the terminal internal carotid artery (TICA), M1, and M2 segments of the middle cerebral artery with the possibility of including tandem occlusions; (4) Alberta Stroke Program Early CT Score (ASPECTS) of 6 or better overall, whereas all evaluations were assisted by a machine learning-based software package (e-ASPECTS, Brainomix, Brainomix Limited, Oxford, UK); and (5) fulfilling DWI or CTP Assessment With Clinical Mismatch in the Triage of Wake-Up and Late Presenting Strokes Undergoing Neurointervention With Trevo (DAWN) [7] or Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke 3 (DEFUSE 3) [8] eligibility criteria on wake-up stroke and 6 to 24 hour treatment window.
All patients signed informed consent forms, including statements about the use of data for research, before treatment was carried out. Intracranial occlusion was treated first (the aspiration catheter was embedded in the clot with the aim of catching the whole clot at once) unless the carotid stenosis prevented it, forcing cervical angioplasty prior to intracranial navigation of the distal aspiration catheter. Preferred endovascular technique employed for intracranial reperfusion was distal aspiration. Our team decided to manage the endovascular treatment applying groin local anesthesia as primary option, and if needed subsequent general anesthesia or conscious sedation. Intravenous (IV) fibrinolysis (recombinant tissue plasminogen activator [rtPA]) was administered to elective patients according to local protocol. In case of rtPA administration, 250 mg of IV aspirin was employed at the beginning of the endovascular procedure. In patients who did not receive rtPA, 500 mg of aspirin was administered. Double antiplatelet therapy with orally administrated clopidogrel or intraarterial abciximab decision during procedure, was made according to individual basis of neurologist and neuroradiologist. The angiographic collateral grade was evaluated according to the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology Collateral Flow Grading System on baseline angiography [9]. Once intracranial recanalization was achieved, treatment of the internal carotid artery (ICA) lesion was left to the discretion of the operator, taking into account residual cervical occlusion/severe stenosis or thrombus apposition after waiting for 30 minutes after intracranial recanalization achievement. In case of platelet aggregation or plaque instability, stent was placed, waiting for another 20 minutes, to monitor new complications in the vascular endoprosthesis, in which case intraarterial abciximab was administered to resolve stent-associated thrombus formation (from 2 to 8 mg according to interventionist criteria). The procedure was terminated once stability in stent patency was achieved. Demographic, radiologic, and clinical data were collected in the prospective A Registry for Thrombectomy In Stroke Therapy from Andalusia (ARTISTA) registry. Successful recanalization (modified thrombolysis in cerebral ischemia [mTICI] grade 2b–3), symptomatic intracranial hemorrhage (sICH) and favorable outcome at 90 days (defined by an mRS ≤2) rates were analyzed according to intracranial occlusion: TICA, M1, and M2. The mRS was evaluated for patients with completed follow-up.
Among 784 included patients (from January 2017 to January 2019), 22.1% (n=174) had TL. Regarding TL subgroups, 20.1% (n=36) had ICA+TICA occlusion, 50% (n=87) ICA+M1 occlusion, and 29.3% (n=51) ICA+M2 occlusion. Baseline characteristics of general sample, TL versus no TL and according to intracranial occlusion are shown in Supplementary Tables 1 and 2.
We found no differences in clinical outcomes at 90 days among TL versus no TL patients. According intracranial occlusion site, ICA+TICA, ICA+M1, and ICA+M2 showed non statistical differences in clinical outcomes at 90 days compared to isolated intracranial TICA, M1, and M2 occlusion (Figure 1). Regarding successful recanalization, sICH, and death rates we did not find statistical differences among subgroups (Figure 2). After stroke subtype adjustment, we found higher rates of successful recanalization (TICI 2b–3) in atherothrombotic TL patients (P=0.025). However there were no differences in mRS 0–2 at 90 days, death and sICH rates among TL versus no TL according to stroke subtype (Supplementary Table 3). Intracranial hemodynamic compensation rates according to site occlusion is specified in Supplementary Table 4.
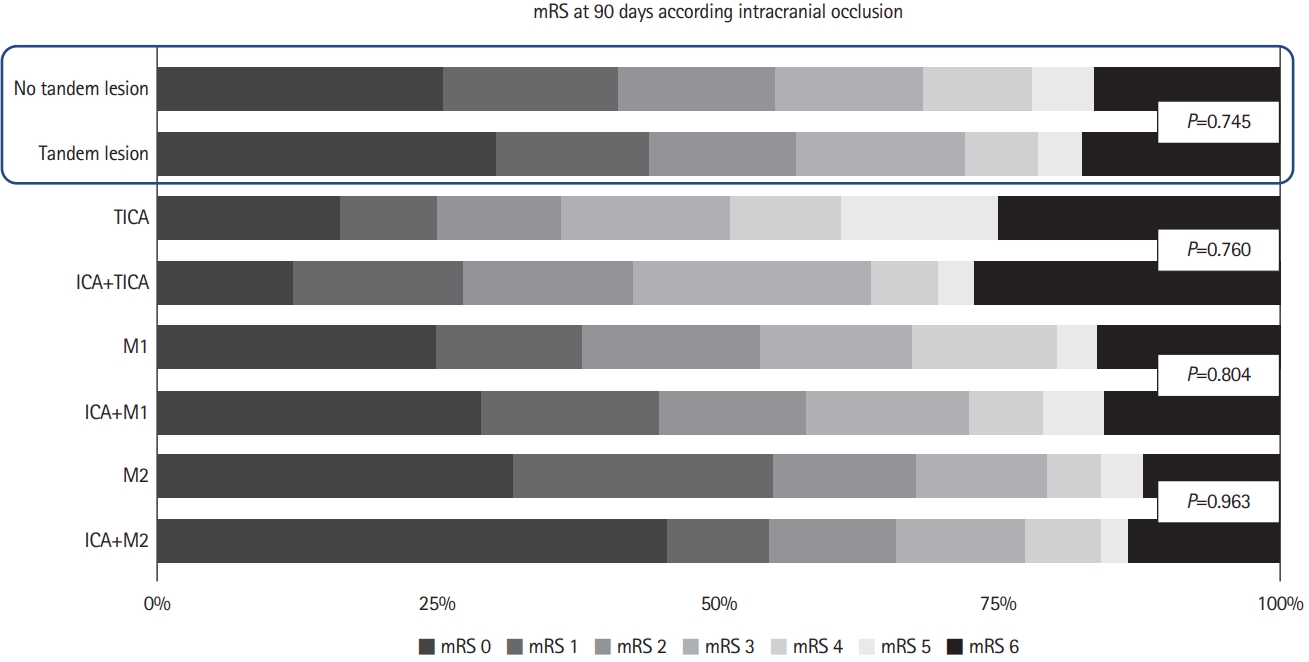
Modified Rankin Scale (mRS) at 90 days in tandem lesions versus no tandem lesions patients and according to intracranial occlusion. TICA, terminal internal carotid artery; ICA, internal carotid artery.
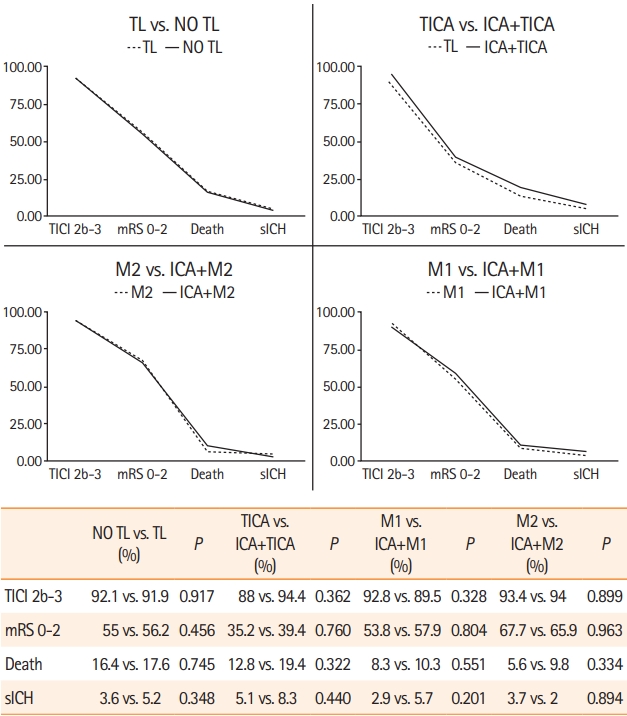
Safety and efficacy mechanical thrombectomy results in tandem lesions (TLs) versus no TLs patients and according intracranial occlusion. TICI, thrombolysis in cerebral ischemia; mRS, modified Rankin Scale; sICH, symptomatic intracranial hemorrhage; TICA, terminal internal carotid artery; ICA, internal carotid artery.
Due to technical complexity of endovascular treatment of TL, this subgroup of stroke patients has been mostly excluded from the clinical trials [2]. Nevertheless, there is a growing evidence about efficacy and safety of MT in TL [10,11], with ranges of 75% to 87% of successful recanalization, 44% to 73.3% of mRS at 90 days, 11% to 16% of mortality, and 4.4% to 8% of sICH [6,10,11]. Antiplatelet therapy, endovascular approach, and stent placement in TL are still uncertain [12].
There are a few recent studies related to MT results according to intracranial occlusion site [13,14], but most of them do not include TL, which is the originality of this manuscript. Ninety days functional outcomes showed non statistical similar results when comparing TL and no TL, which is consistent with results according to intracranial occlusion, downplaying importance to extracranial occlusion, whose clinical impact would be limited. In addition, it is remarkable the better results in more distal occlusions (TICA<M1<M2) in both TL versus no TL. Furthermore, it’s relevant that notwithstanding longer groin-recanalization times in all TL patients, this does not impact negatively on functional outcomes of mentioned patients. The hemodynamic compensation of the Willis circle could involve the difference between TL and no TL. In our cohort, tandem occlusion patients, have shown better intracranial compensation (retrograde ophthalmic artery, anterior communicating artery, and posterior communicating artery function), but no collateral circulation. This finding could explain that despite a longer time to obtain the successful recanalization in TLs, its functional results do not worsen and are even similar to isolated intracranial occlusions. In addition, progressive changes in intracranial hemodynamic compensation secondary to chronic significant carotid stenosis may allow the hypoperfused brain to better tolerate ischemia. According to our hypothesis, we found that cardioembolic stroke was more frequent in no TL (61.4% vs. 11.8%), in which acute LVO occlusion could associate worse Willis circle compensations compared to TL progressive hemodynamic changes. Main limitations of our study are the small number of patients in intracranial occlusion site subgroups and the retrospective evaluation, which introduce a selection bias for patients who met our criteria for MT.
The intracranial treatment approach in the first place proposed in our study, reduces the times of cerebral ischemia and improves the long-term functional result. Lastly, our action protocol guarantees carotid patency at the end of the procedure, since the time necessary to assess intra-stent aggregation or plaque instability is expected after administering the relevant antiplatelet agents. Ensuring cervical patency improves antegrade intracranial flow. Therefore, once intracranial recanalization has been achieved, maintaining correct carotid patency becomes essential to avoid acute reocclusions that waste the initial success.
Our results need further validation in different cohorts; however, we hypothesize that despite being more technically complex and longer procedures, patients with TL have a better tolerance to hypoperfusion, thanks to the intracranial hemodynamic compensation systems associated with a chronic carotid stenosis. This tolerance to hypoperfusion is consistent in the different intracranial occlusion sites despite presenting TLs. TL hemodynamic behavior could set a starting point to study ways of “stroke compensations” in order to reduce ischemia damage.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2020.02747.
Baseline characteristics of the whole sample and according to TL vs. no TL presence
Baseline characteristics of the sample according to intracranial occlusion
Safety and efficacy results in no TL vs. TL after stroke (atherothrombotic vs. cardioembolic) subtype adjustment
Intracranial hemodynamic compensations rates (%) according to site occlusion
Acknowledgements
This project was partially funded by the ISCIII project PI14/00971. The ITRIBiS project (Improving Translational Research Potential at the Institute of Biomedicine of Seville) has the registration number REGPOT-2013-1. Cooperative Cerebrovascular Disease Research Network (INVICTUS+) (RD16/0019/0015).
Notes
The authors have no financial conflicts of interest.
