Introduction
Aphasia is a language disorder that can have profound negative effects on quality of life. It is most often due to left hemisphere stroke but can also be caused by other types of damage—such as trauma or neurodegenerative disease—that affect the language network of the brain. Here, we review therapy for post-stroke aphasia, focusing on recent clinical trials. Although most neurologists have the greatest familiarity with pharmaceutical interventions, we begin by discussing behavioral therapies, because other interventions augment behavioral interventions. That is, medications and noninvasive brain stimulation have been used in conjunction with, rather than in place of, behavioral therapies.
Several recent publications have reviewed the mechanisms of aphasia recovery, and in some cases the mechanisms of therapy [1-4] revealed by changes in task-related brain activations or changes in functional connectivity within functional networks [1-6]. Therefore, we will emphasize the empirical effects of therapy with less focus on the neural mechanisms that promote treated recovery from aphasia.
Behavioral interventions
Behavioral therapy to improve language in stroke aphasia: the basics
By far, the most common approach to aphasia rehabilitation is behavioral speech and language therapy (SLT). SLT was even described by Paul Broca in his seminal 1865 paper [7], and it remains the standard of care for patients with aphasia [8].
There are many different approaches to SLT, with two main camps: (1) Impairment-based approaches focus directly on decreasing the language impairment through structured therapy that targets the sub-components of language such as phonology, lexical-semantics, or syntax [9-11]. The goal is to improve language functions with the assumptions that doing so will generalize to communication abilities and, by extension, communicative quality of life. (2) Functional communication approaches more directly target communication abilities and do not necessarily focus on generalization to reduce speech or language deficits. Rather, these latter approaches are more likely to focus on stimuli with direct personal relevance. Additionally, functional therapy emphasizes eliminating communication barriers in the environment, caregiver training to enhance communication, and improving the success of communication rather than reducing impairment (see Martin et al. [12] for review and examples of the two approaches).
Efficacy of behavioral speech and language therapy for aphasia
The literature on aphasia therapy is marred by mostly single-case design and small group studies (e.g., references [13-15]). Perhaps because of this historical focus on underpowered studies, the efficacy of aphasia therapy was debated for decades and, until relatively recently, remained highly controversial [16-18]. Furthermore, by focusing solely on speech and language measures as markers of outcome, most studies failed to demonstrate meaningful improvements. In acute patients, it has been relatively difficult to demonstrate that early aphasia recovery is spurred by SLT rather than inherent recovery processes [19]. In addition, no standards have been developed for what works best for patients with different aphasic impairment patterns. However, in the past decade, a much clearer picture of the overall efficacy of SLT for aphasia has emerged. Meta-analyses of a large number of smaller studies generally support the notion that SLT is very much worthwhile for aphasic patients [16,17,20,21]. Moreover, the results of a recent Phase III randomized controlled trial (RCT) by Breitenstein et al. [18] unequivocally support the use of impairment-based SLT to improve speech production in chronic aphasia. Specifically, this trial revealed that SLT does improve effectiveness of verbal communication measured using the Amsterdam-Nijmegen Everyday Language Test A (ANELT-A [22]; A-scale [Cohen’s d=0.58; medium effect size]). Moreover, the trial also revealed an improvement in communicative quality of life (Cohen’s d=0.27), an important finding that had eluded many earlier trials (e.g., references [23-25]). Taken together, large meta-analyses and the Breitenstein et al. [18] trial provide robust evidence in favor of SLT for post-stroke aphasia.
Optimal timing of intervention: chronic vs. acute phase of post-stroke
In spite of progress in the field, the optimal timing of intervention after stroke remains unclear. Equally important, it is not known if or when patients reach a recovery plateau. Whereas animal models of stroke suggest early intervention is probably important [26] we have no definitive data in aphasia [27]. Although most aphasia therapy studies have enrolled chronic patients, it seems likely that earlier aphasia therapy is also effective [28]. However, two large RCTs published in the last 5 years do not support this notion. In the Rotterdam Aphasia Therapy Study-3 (RATS-3) [29], 152 patients with acute aphasia due to stroke were randomized to receive either 4 weeks of impairment-based SLT (1 hour/day) or no therapy. Relying on the same outcome measure as Breitenstein et al. [18], ANELT-A [29], no group differences were revealed at the primary endpoint (P=0.805), measured at 4 weeks after randomization, or at secondary endpoints at 3 (P=0.767) and 6 (P=0.807) months after randomization. A more recent RCT by Godecke et al. [30] titled Very Early Rehabilitation for SpEech (VERSE) also yielded a non-significant result for the primary analysis. Among 246 patients with acute aphasia, there were no differences in changes in overall aphasia severity between the three study arms (P=0.59): usual care, usual care+20 SLT sessions, and usual care+20 sessions of specialized impairment-based therapy program designed by an “aphasia Expert Advisory Committee.” The mean number of therapy hours in the ‘usual care’ group was 9.5 (standard deviation [SD] 7.6) over 28 days whereas the other two groups averaged 22.7 hours of therapy (SD 8.4) over 32 days. Although VERSE did not include a ‘no therapy’ control arm, it suggests that increasing the intensity of SLT from a mean of 0.34 to 0.71 hours/day in the early phases of recovery may not further improve recovery from aphasia. If there is a dose-response relationship between the amount of therapy and early recovery from aphasia, one would expect that the more intensive groups would have experienced better outcome. In this context, it is worth noting that early aphasia recovery is highly variable and is probably influenced by several different factors that may be difficult to control in rehabilitation studies. Equally important, it is clear that not all SLT approaches are equally potent and that future trials employing different kinds of SLT may reveal positive outcomes. Moreover, future research may identify the minimum dose needed to affect language improvement in acute to subacute stroke. Just like there is considerable variability in response to SLT [31], it seems reasonable to suggest that some patients may need more therapy to experience an improvement in communication abilities.
In contrast to early aphasia therapy, there is ample evidence, including Breitenstein et al. [18], that more SLT in the chronic phase is associated with greater long-term recovery. A recent study revealed that among chronic stroke patients, improvement in language processing over several years in language processing among chronic stroke patients with aphasia is associated with more SLT sessions [32]. This study emphasized that approximately half of chronic stroke patients continue to experience recovery from aphasia even many years after stroke. The remaining half either are relatively stable or actually experience decline, an often overlooked fact that may represent an under-appreciated therapeutic target. Two earlier studies very much echo the finding that approximately half of chronic patients continue to experience recovery [33,34]. Reasons for decline in language by other patients remain unclear, but white matter hyperintensities seen on T2-magnetic resonance imaging (MRI) or fluid attenuated inversion recovery may be one culprit. One study revealed that patients who experienced long-term language decline were more likely to show greater extent of white matter hyperintensities in comparison to patients who stabilized or improved [35]. A related study showed that these white matter hyperintensities are disproportionally associated with loss of long-range white matter fibers, which could provide a more specific mechanistic account of worsening aphasic symptoms in chronic patients [36]. Although some studies indicate that on average, stroke patients show steady cognitive decline after stroke [37], a careful review of the literature indicates that the average slow decline instead reflects a sharp decline by about 25% of patients, but slow improvement or stability in others. The finding that approximately a quarter of individuals with post-stroke aphasia are at risk for significant decline in function emphasizes the need for more aggressive monitoring of, and therapy for, vascular risk factors among these patients.
Predicting the outcome of behavioral therapy of aphasia
Although demonstration for the efficacy of SLT for aphasia is improving, predicting which patients are likely to respond and what therapy works best for individual patients remains a major challenge. In 1972, Darley [38] suggested that factors such as age, education, intelligence, social status, health, time post-stroke, and aphasia type are related to success in aphasia therapy, but that very few studies had actually incorporated these factors as predictors of outcome. Unfortunately, more than four decades later, the situation is not drastically improved, as research has failed to establish a strong relationship between specific patient factors and treated aphasia recovery. A few studies have related patient characteristics to spontaneous recovery [14,39-41]; however, it is not known whether the same factors would predict aphasia therapy outcome. Accordingly, clinicians who routinely treat patients with aphasia have somewhat limited empirical data to guide their therapy and predict outcome. Whereas biomarker studies are common in medicine, somewhat limited research has focused on understanding the relationship between patient factors and aphasia rehabilitation potential in stroke (e.g., references [40,42,43]). Some recent studies suggest imaging markers, such as larger lesion volume [44,45] and greater severity of leukoaraiosis [35], are associated with worse long-term outcome. Other factors have also been implicated such as the degree of damage to posterior superior temporal gyrus or arcuate fasciculus [46,47], independently of lesion volume, and recovery in patients with damage to these critical areas may be influenced by medications [46].
Aphasia severity, a factor related to overall lesion volume, is one of the very few variables that has been identified as a reliable predictor of SLT outcome, and it is generally accepted that more severe patients are less likely to respond to SLT [41,48]. The aforementioned trials by Breitenstein et al. [18], Nouwens et al. [29], and Godecke et al. [30] each revealed that aphasia severity was a strong predictor of overall outcome with more severe patients benefitting less from SLT. It is a caveat; however, that aphasia severity is a multidimensional construct where different patients with severe aphasia might present with very different language impairment profiles. Nevertheless, all else being equal, patients with more severe language impairment are probably less likely to experience spontaneous or therapy-induced recovery. Importantly, speech-language pathologists seem to be very much aware of the connection between aphasia severity and treatment outcome. In a survey of 54 speech-language pathologists, severity and nature of post-stroke aphasia were identified among the most important factors for forming an aphasia prognosis [49]. We will return to this aphasia severity and treatment outcome in more detail below.
In a trial that recently completed enrollment (n=128) titled Predicting Outcomes of LAnguage Rehabilitation in aphasia (POLAR) [50] some initial results are emerging, identifying factors that influence SLT-related improvements in naming and discourse production at 1-week, 1-month, and 6-months following SLT completion. POLAR involves a cross-over design where half of the participants with chronic aphasia are randomized to first undergo 3 weeks of SLT (5 times/week) focused on phonological processing and then receive 3 weeks of semantically focused SLT with a 4-week break between the two SLT phases. The remaining half of participants are randomized to receive semantic SLT first and then phonological SLT during the second therapy phase. Preliminary analyses incorporating data from the first 74 trial completers revealed statistically significant improvements in correct naming on the Philadelphia Naming Test (PNT) [51] when comparing results between baseline and 1-week after completion of the second therapy phase (t(74)=6.1765, P<0.00001). This effect was maintained at 6 months after therapy completion (t(74)=5.6, P<0.0001). In addition, significant improvements were revealed in discourse production immediately after completion of the second therapy phase (t(74)=2.37, P=0.02), and again at 6 months post-therapy (t(74)=3.23, P=0.002). Discourse production was qualified and measured as total words produced per minute during three separate discourse tasks.
In accordance with the primary purpose of POLAR, linear mixed effects modeling was applied to understand what baseline factors predict therapy outcome at 6 months post-treatment. For change in correct naming, two predictors emerged: Overall aphasia severity (P<0.03) and participant age (P=0.006). Overall, older individuals and those with more severe aphasia were less likely to show treatment related improvements in the POLAR trial. A significant relationship between a measure on a cognitive test that is thought to minimally tax with involve language processing, the WAIS matrix test and treatment outcome in POLAR was not revealed, which is inconsistent with what others have found. For example, Dignam et al. [52] revealed that cognitive factors such as verbal working memory predict SLT-related improvements in naming. Similarly, a study by Gilmore et al. [53] suggested that cognitive factors predict the outcome of SLT targeting either sentence comprehension or naming in aphasia. Much like Breitenstein et al. [18], Nouwens et al. [29], and Godecke et al. [30], these preliminary data from POLAR suggest participants with more severe aphasia are less likely to respond to SLT. This point is illustrated in Figure 1, which includes data from the POLAR trial showing the relation between WAB-R aphasia quotient (WAB-R AQ), and improvements in correct naming on the PNT from baseline to 1-week post-therapy. The WAB-R AQ is a measure of overall severity, and the therapy outcome here was calculated two different ways (1) raw change in correct naming and (2) change in correct naming divided by the room for improvement at baseline. These data suggest that although milder aphasia does not guarantee positive therapy-related improvement in naming, the most severe patients are considerably less likely to respond. As a side note, overall lesion size was not associated with outcome at 6 months post-therapy in POLAR.
Another POLAR report showed that the type of SLT matters for different individuals with aphasia. Again, POLAR involves a cross-over design aimed at comparing the effects of phonologically focused SLT to semantically focused SLT on speech production in aphasia [54]. Regardless of therapy order, the average improvement in naming following the semantically focused SLT was far superior to the outcome of the phonologically focused SLT (P=0.008). In spite of the clear overall advantage of the semantically focused SLT, it is worth noting that different patterns of therapy response emerged across the two therapy types. Some participants responded to both approaches, some responded to only one and several more showed no response, regardless of SLT type. Crucially, there was an interaction between aphasia severity and therapy type response, where more severe individuals with less fluent speech responded more to phonological therapy (P=0.049), whereas milder cases showed significantly better response to semantic therapy (P=0.018). This relationship is clearly demonstrated in Figure 2, which shows the correlation matrix between naming improvements following the semantic or phonological therapies in relation to various measures of language abilities. Specifically, positive response (improvement in naming) to the phonologically focused SLT was negatively correlated with various language scores, including measures of grammatical and phonological processing as well as measures of overall aphasia severity and naming impairment. In contrast, response to the semantically focused SLT was positively correlated with the same measures of language abilities. With regard to aphasia type, individuals with either anomic or conduction aphasia showed preferentially better response to the semantic than the phonologically focused treatment. However, no aphasia type was associated with significantly better response to the phonologically focused treatment over the semantic treatment.
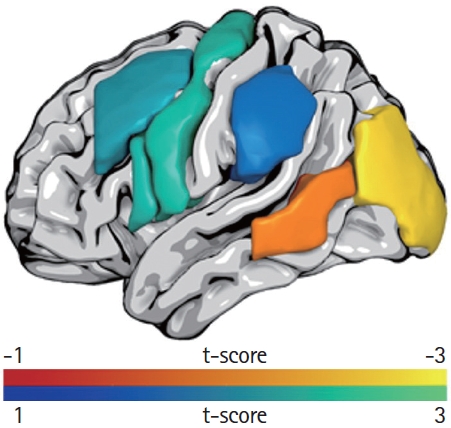
To explore whether lesion location predicted outcome in POLAR, lesion-load in language related cortical regions [55] was used to predict improvement on the PNT (n=77) in univariate analyses. For overall change in correct naming on the PNT from baseline to 1-week after completion of the second treatment phase, greater proportional damage to the left middle occipital gyrus (ß=-0.294, P=0.010) and the posterior middle temporal gyrus (ß=-0.216, P=0.060) predicted poorer response to treatment (Figure 3). Response to phonological treatment was independently predicted by damage to four regions of interest: the middle frontal gyrus (ß=0.232, P=0.042), inferior frontal gyrus (IFG) pars opercularis (ß=0.255, P=0.025), precentral gyrus (ß=0.260, P=0.022), and supramarginal gyrus (ß=0.199, P=0.083). Interestingly, aphasia severity did not emerge as a significant predictor when damage to these four regions was accounted for. No single lesion location predicted response to semantically focused treatment. This preliminary report from POLAR suggests that lesion location is actually associated not only with overall SLT response but also with SLT type. It is too early to say whether these results can be used to guide clinical treatment of aphasia but, at the very least, they provide insights into what kinds of lesion patterns are associated with poor treatment outcome.
There are certainly many other ways that SLT can be varied other than contrasting semantic versus phonological training. For example, Conroy et al. [56] compared SLT that emphasized quicker naming to a more conventional approach where speeded naming was not emphasized. In short, this study revealed that greater focus on faster speed and greater accuracy during training was more effective for improving naming accuracy and speed of naming for trained items as well as for generalization to connected speech. Along with Basilakos et al. [35], the findings by Kristinsson et al. [54] emphasize the importance of personalized predictors of therapy outcome where baseline factors can be used to stratify SLT type.
In a retrospective analysis, our group (unpublished) examined data from three separate trials to understand the relationship between overall aphasia severity, age, and aphasia therapy success. Although the three studies involved different kinds of aphasia therapy and different sample sizes (total n=179), the outcome measures for each trial were similar and focused on changes in correct naming abilities. Across all three studies, overall aphasia severity, measured as the WAB-R AQ, was a strong predictor of therapy outcome. Specifically, individuals with more severe aphasia were less likely to respond to SLT. In two out of the three studies, age was also related to outcome, such that older participants were less likely to respond to therapy. Given that greater aphasia severity is, in general, related to larger lesion size, it seems possible that lack of improvement among more severe patients is related to less residual cortex that can take over the function that was lost. Consistent with previous research by Bonilha et al. [57], greater sparing of the residual language network is related to better SLT response. The relation between younger age and better SLT response is certainly not surprising given that any kind of speech and language restoration associated with SLT must be related to either functional or structural plasticity, or both. As has been shown in numerous studies, the extent of brain plasticity decreases steadily with age [58-60], which may explain why older patients are somewhat less likely to respond to SLT. However, it is imperative to note that although age was related to outcome, it was a relatively weak predictor compared to overall aphasia severity. Indeed, many participants older than 65 in the aforementioned studies did respond to therapy even though their younger counterparts, as a group, tended to benefit even more.
Optimal dosage for aphasia therapy is an area of emerging focus in the field of aphasiology. A comprehensive review of this issue found insufficient evidence to recommend an optimal dosage that would result in maximum treatment outcome [61]. Although it seems highly plausible that more aphasia therapy is better in almost any case of aphasia, treatment dose as a prepredictor of outcome remains under-studied. For a comprehensive review of this issue, readers are referred to Harvey et al. [61].
Individual therapy vs. group therapy
The most common mode of SLT is one-on-one delivery where a single clinician, typically a speech-language pathologist, treats a single patient. Another mode of SLT delivery is group therapy, a tradition that was started as early as post World War II in Veterans Administration hospitals in the United States. Very limited data are available on the effectiveness of group SLT for aphasia, and third party reimbursers tend not to support it [62]. Regardless of the mode, most persons with chronic aphasia do not receive an adequate amount of aphasia therapy to maximize recovery. Two sources of evidence are particularly important in this context: First, as discussed above, considerable evidence supports the utility of SLT for aphasia [16-18]. Second, some studies show that more therapy is better, regardless of intensity (the amount per time unit) [63,64]. Unfortunately, recent data on the typical number of aphasia therapy sessions are lacking. One survey published 20 years ago indicated that patients with aphasia in the United States could expect to receive approximately 15 sessions of therapy [65]. It is unlikely that the average number of therapy sessions has increased in the current healthcare climate. Thankfully, the number of aphasia centers that provide more intensive therapy and aphasia groups is on the rise (e.g., references [66-68]). Yet, the number of individuals served by these centers represents a very small fraction of the two million who have chronic aphasia in North America [69]. There are several reasons why individuals with chronic aphasia are underserved. Lack of third party reimbursement is undoubtedly a major barrier [69]; however, other factors such as difficulty with transportation to and from therapy and limited availability of speech-language pathologists, especially in rural areas, may also play a role.
In-person vs. telerehabilitation
One way to improve access to aphasia therapy is by means of telerehabilitation (telerehab). Providing SLT via remote therapy could vastly increase the availability of speech-language pathologists and possibly decrease costs. A few studies have started to explore the effectiveness of telerehab for aphasia, both clinician-supervised therapy and unsupervised therapy (computerized therapy). One recently completed pilot trial indicated that supplementing ‘standard of care’ SLT for aphasia with telerehab did not improve the primary outcome (naming) compared to ‘standard of care’ SLT by itself [70]. It is worth noting; however, that this trial was not aimed at establishing whether telerehab by itself is non-inferior to traditional in-person SLT. A separate trial compared the effects of telerehab and in-person SLT in individuals with chronic aphasia and found no difference between the two modes of therapy [71]. Unfortunately, this study included only five participants in each condition and, therefore, was almost surely underpowered. A larger study [72] (n=44) compared in-person SLT to a hybrid version of telerehab SLT: one session per week of clinician administered therapy via telerehab, supplemented with computerized therapy (Lingraphica) [73] at least four times a week. This trial found hybrid telerehab to be non-inferior to in-person SLT for chronic aphasia. In a separate study, Telerehabilitation Group Aphasia Intervention and Networking (TeleGAIN) [74], 19 participants with chronic aphasia underwent group treatment for 12 weeks that was delivered via telerehab. Here, treatment focused on conversational engagement among participants with aphasia as well as functional treatment approaches. Although this study assessed a large number of endpoints, its most notable result was that participants communicative quality of life was improved after treatment compared to baseline. This is a very promising finding, which can be further tested in a larger trial that includes a control group and where the statistical analyses control for multiple comparisons when calculating changes across outcome measures.
Larger studies are needed to show that telerehab is non-inferior to in-person SLT, which will allow expanded availability of aphasia rehabilitation. The recent coronavirus disease 2019 (COVID-19) pandemic has forced many clinicians to transition to telerehab in place of face-to-face delivery, and third party payors have begun to cover the charges, which suggests a potential for this type of service delivery model to expand access to SLT for aphasia.
Computerized therapy
A fast-emerging approach to aphasia management is computerized SLT. One group study (n=21) that employed a pre- and post-comparison of naming abilities showed a positive outcome of a 6-month SLT regimen [75]. Much like many other trials, this study also showed a relationship between aphasia severity and long-term outcome with more severe patients maintaining less improvement at 4 months post-therapy. The authors concluded that independent home practice using computerized therapy could represent a viable alternative to clinician-based therapy, which often is not available to chronic patients. A large RCT (n=278) with three arms further supports the potential utility of computerized aphasia therapy (Big CACTUS) [76]. Here, patients at least 4 months post-stroke were randomized to receive usual care; usual care supplemented with self-administered computerized SLT; or usual care with added attention therapy. The group that received the added computerized SLT experienced greater improvement in naming (P<0.0001), but not in functional communication (P>0.05), compared to the other two groups. Although improvement in ‘real life’ functional communication is the desired outcome of aphasia therapy, such improvements are notoriously difficult to measure. Nevertheless, the results provide convincing evidence that computerized SLT may further enhance naming ability in aphasia. Subsequently, Latimer et al.77 analyzed the Big CACTUS data to understand whether adding self-administered computerized SLT to usual care was cost-effective. Unfortunately, because Big CACTUS did not yield a statistically significant improvement in quality of life associated with the addition of computerized SLT to usual care, the cost-effectiveness analysis was inconclusive. Nevertheless, this study emphasizes the need for other trials to follow suit and build in cost-effectiveness analyses so that stakeholders can understand not only whether a given treatment is effective in improving communication abilities but also whether it is cost-effective.
A more recently completed trial by Fleming et al. [78] relied on a cross-over design to study the effects of computerized therapy to improve auditory comprehension in aphasia. Using an in-house developed app (Listen-In) that targets auditory comprehension at the single word, phrase, and sentence level and is run on a computer tablet, participants (n=35) were randomized to first receive 12 weeks of self-administered Listen-In training and then 12 weeks of usual care, or vice versa. One of the several strengths of this trial was that participants received a relatively high dose of therapy with an average of 85 hours of training using Listen-In. A study-specific outcome test of auditory comprehension revealed greater comprehension improvement for trained items associated with Listen-In in comparison to usual care (t(34)=4.09, P<0.001, Cohen’s d=1.32).
A similar comparison for untrained items revealed non-significant results. Changes in auditory comprehension measured on two sub-tests of the Comprehensive Aphasia Test [79] were also not significant between Listen-In and usual care. Although not the purpose of the current review, it is notable that Fleming et al. [78] also revealed structural brain changes (assessed using T1-MRI) in both white (left hemisphere) and gray matter (right hemisphere) superior temporal gyrus that correlated with therapy outcome in a sub-set of the participants (n=25). Although these effects need to be verified in a larger sample, this study provides convincing evidence that aphasia therapy changes the structure of the brain. Most importantly for clinical purposes, the study by Fleming et al. [78] demonstrates that computerized therapy can improve auditory comprehension in aphasia. Other smaller studies also further support the utility of computerized SLT. For example, a study of seven chronic stroke patients with aphasia showed that computerized SLT yielded better language outcomes than a ‘mind-game’ intervention that did not focus on speech or language training [80].
Theory-based therapy
There is a wide range in SLT approaches with regard to which mechanism they tackle or what aspects of language they are expected to improve. For example, most of the studies reviewed so far focus on speech production without necessarily emphasizing which aspect of processing is targeted by therapy. In contrast, Treatment of Underlying Forms (TUF) specifically emphasizes meta-linguistic training to improve grammatical processing, both for speech production and comprehension, in patients with Broca’s aphasia [81]. In short, TUF initially focuses on training lexical properties of verbs and mapping verb arguments. Then, training moves to complex sentence structures with emphasis on integration and reanalysis of semantic roles. One of the keys to TUF is that it trains more complex sentence structures, which has been shown to generalize to simpler structures [82]. Although TUF is not a new therapy approach, a recent study with randomized assignment to either TUF (n=14) or a control group that received no therapy (n=5) provides evidence that TUF improves sentence processing among aphasic patients with agrammatism [83]. Relying on the principles of TUF, training focused on complex sentence structures (long passive sentences with adjunct clauses), which resulted in improvements among the experimental group in both comprehension and production of trained sentences structures, as well as related sentences structures, at both immediate follow-up and at 3 months following therapy completion. No changes in performance were noted in the control group immediately after therapy completion, and no changes in the processing of unrelated sentence structures were noted in the experimental group. Along with several smaller studies [81,84], this study by Barbieri at al. [83] suggests TUF as a preferred approach to treat patients with agrammatic aphasia.
In the past two decades, considerable research has focused on the effects of constraint-induced language therapy (CIAT) for aphasia [85]. This behavioral therapy approach was based on evidence from motor rehabilitation indicating that constraining the spared limb and forcing patients to use the affected limb in therapy resulted in significant motor improvement. Applying this approach to SLT for aphasia, patients are discouraged from using modalities other than speech, such as gestures or writing, to communicate. This approach has shown promise in several trials [86,87], although it is not superior to more conventional SLT provided at similar intensity levels [88,89]. It is possible that the success of CIAT so far is primarily related to relatively greater intensity of therapy in those studies rather than the actual constraining of non-verbal expression. An ongoing RCT (Constraint-Induced or Multi-Modal Personalized Aphasia Rehabilitation [COMPARE]) by Rose et al. [90] may shed light on this issue by comparing the effectiveness of CIAT to multi-modality focused SLT as well as standard of care SLT. Unlike CIAT, the multi-modal SLT and the standard of care approaches do not incorporate the constraining aspects of CIAT, but therapy time is controlled across the three study arms. At the time this is written, enrollment in COMPARE is complete, but the results have not yet been published.
Social approach to therapy
We end this section by discussing what we consider a very positive development to managing aphasia: Life Participation Approaches to Aphasia (LPAA). Although LPAA is not a new concept, it has risen in popularity with annual conferences and workshops. Unlike impairment-based or functional therapy approaches to aphasia therapy that focus primarily on reestablishing communication abilities, LPAA emphasizes reintegration of persons with aphasia into the community by focusing on residual personal strengths instead of communication disabilities. LPAA could be regarded as an umbrella term for different tactics and approaches that maximize life participation. For example, training where individualized communication strategies are identified and practiced by the person with aphasia and communication partners can be effective [91-93]. Along the same lines, testing for what individuals can, rather than cannot, communicate may help identify residual language abilities and potential communication strengths that otherwise might go unnoticed [94]. Another important development in the LPAA realm is the increasing number of aphasia groups and aphasia centers [67,95,96]. Participation in aphasia groups and centers not only provides psycho-social benefits but may also improve communication and language [97]. Despite the lack of large trials of the utility of LPAA, efforts focused on reintegration of persons with aphasia into society should be considered in very positive light. This brings us back to the issue of severity and the fact that persons with the most severe aphasia are unlikely to respond to SLT. We suggest that patients with the most severe aphasia, and, as a result, the least likely individuals to respond to SLT, may be best served by focusing more on LPAA, including counseling, and improving communication via approaches such as conversational coaching [98], training alternative communication modalities [99], or even alternative or augmentative communication devices [100,101]. This notion is developed in more detail below.
Several large RCTs of SLT in aphasia provide evidence that patients with more severe aphasia are less likely to respond to therapy [18,29,30]. This effect is very salient and is demonstrated in Figure 1 where participants with the most severe aphasia in the POLAR trial experienced very minimal improvement in speech production following 30 sessions of SLT. We suspect that the patients most likely to be referred for SLT are the most severe patients. Unfortunately, these individuals are the least likely to respond to SLT compared to their counterparts with moderate or mild aphasia. Based on this evidence, it would be tempting to discourage referrals of the most severe patients for SLT and, instead, focus rehabilitation on the less severe cases who are more likely to be responders. We think this would be a mistake as we believe all persons with aphasia would benefit from speech therapy services, but that these services need to be personalized based on the individuals’ impairment profile, communicative needs, and recovery potential. In Figure 4, we suggest a simple rubric for how severity of aphasia might be used to determine how rehabilitation efforts could be best tailored to the needs of individuals patients. For the purpose of illustration, we have arranged aphasia severity levels from left-to-right starting with the mildest cases of aphasia, which usually involve relatively smaller cortical lesions, and progressing to the most severe cases on the right, which tend to have the most extensive cortical lesions. The graphs represent hypothetical language impairment scores for speech production, comprehension, reading, and writing. The bottom of the figure represents a proportional continuum between impairment or functional based therapy versus LPAA or counseling. The patients with the most severe aphasia, including those with global aphasia, would focus mostly on LPAA with relatively little effort on SLT. Those with less severe aphasia, primarily in the middle of the range between very severe and mild aphasia, would focus relatively less on LPAA but more on direct SLT using either impairment-based or functional SLT approaches. The patients with the least severe aphasia would focus almost all of their effort on direct SLT. Crucially, the type of rehabilitation and level of counseling would need to be further personalized within different levels of overall severity to maximize benefit. This last point cannot be overstated as overall severity levels can mask substantially different patterns of language impairment, personal needs, and goals among patients who score in the same severity range on aphasia tests.
Pharmaceutical interventions
Acute interventions
In acute ischemic stroke, the primary mechanism of recovery is restoration of blood flow to the penumbral tissue surrounding the core infarct. Numerous large RCTs have shown overall benefit in outcome with intravenous thrombolysis [102] or endovascular therapy [103,104]. While none of these trials have specifically evaluated the effects on language, case series studies have shown that such interventions do result in improvement of language functions [105]. One small RCT also showed that temporary elevation of blood flow to improve perfusion early after left hemisphere stroke due to large vessel occlusion or stenosis was associated with language improvement [106].
Pharmaceutical interventions to designed to augment behavioral language therapy for aphasia
No study has provided evidence that pharmaceutical intervention results in significant improvement in language in chronic post-stroke aphasia in the absence of SLT [107]. However, several trials have provided preliminary or inconsistent evidence that some medications may augment the effects of SLT, as reported below. A plausible mechanism is that language recovery depends on neuroplasticity—neural networks must be modified either by changing connectivity between undamaged nodes of the residual language network or by incorporating new nodes or other networks to assume the functions of the damaged ones [108,109]. There is evidence from both humans and animals that behavioral interventions such as mass practice can bring about this type of reorganization through long-term potentiation and long-term depression. This type of neural plasticity is facilitated by the presence of neurotransmitters such as norepinephrine, acetylcholine, serotonin, and dopamine [110,111]. Therefore, medications that alter the availability of these neurotransmitters could enhance neuroplasticity [112]. While most of the trials of medications to enhance recovery have focused on motor recovery, several RCTs have evaluated the effects on language recovery.
Early RCTs evaluated the effects of sympathomimetics, which elevate brain catecholamines. A few small nonrandomized trials (see Llano and Small [113]) and one larger RCT [114] demonstrated small but statistically significant effects of dextroamphetamine in augmenting language therapy to improve language test scores. However, results were not adjusted for differences in language therapy duration and have not been subsequently replicated. Levadopa and bromocriptine have shown no consistent benefit over placebo in small RCTs [115-117]. Likewise, small RCTs of cholinesterase inhibitors have shown only scant and/or short-lasting beneficial effects on language compared to placebo [118,119]. Memantine, a noncompetitive antagonist of the N-methyl-D-aspartate (NMDA) receptor, with effects on serotonin and dopamine receptors and potential reduced excitotoxicity [120], has shown similar minor and short-lived positive effects in one open-label study, which has not been replicated in RCTs [13]. Finally, selective serotonin reuptake inhibitors (SSRIs), which have been shown in RCTs to have a positive effect on post-stroke motor recovery measured with the Fugl Meyer scale [121] and greater improvements on a cognitive battery [122], but no effect on the less sensitive modified Rankin Scale [123,124], have not been studied in RCTs for aphasia recovery. In summary, there is no consistent evidence that any medication has a substantial effect on aphasia recovery, despite numerous small RCTs to evaluate potential benefit conducted over the past five decades.
Noninvasive brain stimulation
Another approach to augmenting synaptic plasticity and reorganization of the networks that support language is through noninvasive brain stimulation. The two modalities that have been evaluated in RCT are transcranial direct current stimulation (tDCS) and repetitive transcranial magnetic stimulation (rTMS).
tDCS
The mechanisms underlying the effects of tDCS on function are not entirely clear, although studies have shown that application of weak (1 to 4 mA) continuous electrical current to the scalp induces a subthreshold polarization of cortical neurons beneath the stimulated area. The polarization is too weak to generate an action potential, but “primes” the network that is stimulated by the concurrent behavioral task, altering their excitability [125]. In this way, tDCS paired with a behavioral task can augment short- and long-term synaptic plasticity. Studies in animals and humans have provided evidence that tDCS effects depend on a brain derived neurotrophic factor (BDNF)-dependent mechanism [126,127]. While the influence of the current on polarity of neurons is relatively widely distributed, functional MRI studies reveal that the entire network activated by the concurrent task is modulated when tDCS is applied to any part of the network [128], but the excitability is specific to the task-related network. Not surprisingly, then, tDCS applied in isolation (without concurrent language task) has no effect on language function in participants with aphasia [129].
Double-blind RCTs of tDCS use a sham control that participants are unable to distinguish from real tDCS. The sham consists of 30 seconds of 1 to 2 mA stimulation which is faded to 0, mimicking the sensation of continuous (e.g., 20 minutes) 1 to 2 mA stimulation, which is generally perceived for only the initial 20 to 30 seconds [129]. RCTs in the last 5 years have evaluated 1 to 2 mA of anodal or cathodal tDCS for 15 to 20 minutes, based on rodent studies by Fritsch et al. [126] demonstrating that 15 minutes of continuous tDCS significantly increases BDNF levels for longer than 1 hour. Anodal tDCS consistently increases the firing rate of targeted neurons, while cathodal tDCS has mixed effects [129]. In humans, over 35 RCTs have been carried out to evaluate anodal or cathodal tDCS (or both) for post-stroke aphasia (see Biou et al. [130] in 2019 for a systematic review). The location of electrode placement, language therapy methods, and number of sessions vary across RCTs, although most have used anodal tDCS over left hemisphere language areas, or anodal or cathodal tDCS over right hemisphere homologues (the latter with the goal to inhibit potentially adverse activity in the right hemisphere), or use left anodal and right cathodal simultaneously.
Most trials of tDCS have been carried out in chronic stroke, and most published studies have shown better language outcomes in the tDCS group or condition relative to the sham group or condition in each of these trials, even though both groups generally show some gains with SLT (with or without tDCS; see recent Cochrane review [131]). For example, one study demonstrated anodal tDCS over the left IFG in conjunction with language therapy resulted in significantly greater improvement compared to sham in picture description (gains of 19.5 vs. 10.6 correct words, P=0.033), noun naming (18.3 vs. 9.2, P=0.024), and verb naming (18.4 vs. 7.3, P=0.019) [132]. Several studies have reported that anodal tDCS (relative to sham) delivered to motor cortex paired with naming therapy results in greater gains in language outcomes [133,134]. The largest (n=74) double-blind RCT of tDCS to augment naming therapy reported significantly greater improvement in accuracy of naming untrained items with tDCS versus sham, each paired with a computer-delivered lexical-semantic therapy for 15 sessions [135]. Improvement in naming was 70% greater in the left hemisphere anodal tDCS group relative to the sham group: a mean increase in accurately named objects from 13.9 (95% confidence interval [CI], 9.0 to 18.7) for tDCS versus 8.2 (95% CI, 3.8 to 12.6) for sham. Furthermore, there was an interaction between anodal tDCS and polymorphism of the BDNF gene [127]. In the group that received tDCS, participants with the normal val/val polymorphism showed greater response to aphasia therapy than the Met allele carriers, whereas corresponding differences were not revealed among participants in the sham condition [127].
Studies of cathodal tDCS to augment SLT have targeted right IFG or right cerebellum. A group of patients who received cathodal tDCS to right IFG with naming therapy showed faster response times in naming compared to the group who received sham plus naming therapy (1.29 to 2.57 seconds, P=0.050) [136], consistent with the hypothesis that inhibiting right IFG may facilitate language [137]. A few studies have used cathodal (or anodal) tDCS to the right cerebellum with language therapy, based on the cerebellum’s role in language and learning and both inhibitory and excitatory connections between the right cerebellum and left language cortex [138-140]. A randomized order, double-blind, sham-controlled within-subject cross-over trial of right cerebellar tDCS showed greater improvements in spelling with tDCS versus sham (both with spelling therapy) on trained (from 0% to 52.5% correct vs. 0% to 97.5% correct, P<0.05) and untrained words (0% to 27.5% correct vs. 0% to 82.5% correct, P<0.01) [138]. Generalization to written picture naming was achieved only after right cerebellar anodal tDCS.
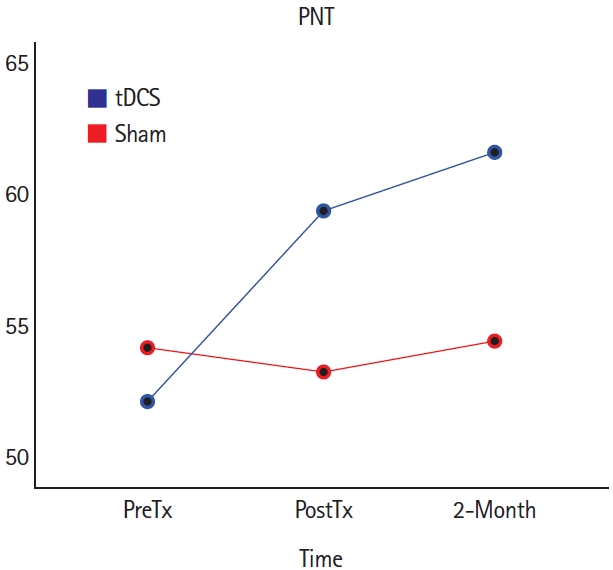
More recent data from a randomized order, double-blind cross-over trial of right cerebellar tDCS versus sham, combined with computer-delivered lexical-semantic therapy in 20 participants with chronic post-stroke aphasia by our collaborators showed significantly improved naming of treated items in both conditions, but improvement in untreated items only during the tDCS condition (Figure 5) [139].
The few sham-controlled studies of tDCS+SLT in subacute post-stroke aphasia have shown modest gains with right cathodal tDCS [141] or no significant effect with just five therapy sessions [142]. However, these studies have shown that 1 to 2 mA tDCS is well-tolerated and safe early after stroke. Additional RCTs in subacute stroke (with more than five therapy sessions) are ongoing.
rTMS
The rTMS is also believed to modulate neuronal activity and enhance neuroplasticity. Unlike tDCS, rTMS can cause action potentials (depending on the frequency), so it can result in changes in network connectivity strength in the absence of a behavioral task. High frequency rTMS is excitatory, while low frequency rTMS (usually 1 Hz) is inhibitory. In trials to improve language, low frequency rTMS is generally applied to the contralesional right hemisphere to inhibit its activation, which is thought to enhance perilesional left hemisphere activation.137 Likewise, high frequency rTMS is generally applied to left hemisphere perilesional areas to enhance activation during language. Most rTMS studies include behavioral language intervention immediately after the stimulation. Most RCTs include a sham group or condition, although the conditions are usually distinguishable by participants who receive both conditions. Therefore, cross-over trials are not generally conducted (but see Rubi-Fessen et al. [143]), as the participants cannot be adequately blinded to the intervention condition. However, randomized, parallel, sham-controlled studies are possible.
In subacute stroke, studies of both low frequency and high frequency rTMS have reported benefits in language recovery. One recent RCT used high frequency rTMS to the left IFG and low frequency rTMS to the right IFG (or bilateral sham) for 10 sessions, each followed by 30 minutes of SLT. The rTMS group showed significantly greater improvements than the sham group in accuracy of word comprehension (P=0.04), naming (P=0.01), repetition (P=0.002), and in aphasia severity (1.8±1.2 vs. 0.9±0.3, P=0.018) [144]. Significant gains in language with rTMS were observed immediately after therapy and 2 months later, and were specific to language. In a pilot, blinded assessor RCT of low frequency rTMS (vs. sham) applied to the right IFG for 30 minutes, followed by SLT for 45 minutes, for 10 sessions in 12 patients, the rTMS group showed higher language scores post-treatment (e.g., 50.3±28.3 vs. 39.3±18.1) on a 100-point summary score, despite similar baseline scores. The authors reported a significant time by group interaction but no P-values or CIs were reported [145]. A larger cross-over study (n=30) of low frequency rTMS (vs. sham) to the right IFG, each with subsequent 45 minutes of language therapy for 10 sessions, also showed greater improvement with rTMS compared to sham on object naming (47.4±28.3 vs. 35.3±30.1, P<0.05), object naming reaction time (12.1±4.9 vs. 13.9±5.1, P<0.01), action naming (34.8±24.6 vs. 25.9±20.4, P<0.01), and action naming reaction time (15.4±5.2 vs. 15.4±5.7, P<0.01) immediately after therapy, and lasting up to 3 months, and showed gains in functional communication [146].
Similar effects of both low and high frequency rTMS have been reported in studies of chronic post-stroke aphasia (see a Naeser et al. [137] for review and Ren et al. [147] for a meta-analysis). One recent study first used functional near infrared spectroscopy to determine which hemisphere showed greater activation during language, to determine the most appropriate frequency of rTMS to right IFG [148]. Patients with greater left hemisphere language activation received low frequency (inhibitory) rTMS to contralesional right IFG and those with more right hemisphere language activation received high frequency (excitatory) rTMS to right IFG. All eight patients received language therapy following rTMS, for 10 sessions. Equally significant improvements in language functions were observed with low and high frequency rTMS to right IFG, but there was no sham control group or condition.
While most rTMS studies for aphasia include language therapy immediately after rTMS, one study investigated concurrent versus subsequent language therapy [149]. They compared SLT immediately following 1 Hz rTMS to right IFG, to synchronous delivery of language therapy with rTMS, and to language therapy alone. Synchronous language therapy was associated with greater improvements in verbal description, object naming, and action naming than either rTMS with subsequent language therapy or sham plus language therapy. The loud clicking noise during rTMS limits the type of language therapy that can be undertaken simultaneously with TMS.
Conclusions
The mainstay of post-stroke aphasia therapy has always been, and continues to be, behavioral SLT. There have been recent innovations to enhance its effectiveness, by increasing the time in therapy (e.g., through language therapy apps), by individualizing therapy to address aphasia severity and specific speech or language deficits, or increasing access to therapy through telerehab. Behavioral SLT has also been augmented with medications or noninvasive brain stimulation methods that facilitate neural plasticity. Functional imaging studies show changes in neural networks supporting language that correlate with improvements. Both animal studies and genetic studies of humans indicate that at least tDCS may work through a BDNF-dependent mechanism. Future studies will likely investigate the combination of pharmaceutical and noninvasive brain stimulation approaches to augment SLT. See Figure 6 for a schematic of how interventions lead to reorganization of the language network.
In spite of progress, a major priority for future research in aphasia rehabilitation is to establish ‘minimum clinically significant differences’ with regard to gains in both language and quality of life. Another important goal is to provide evidence for what therapy works best for patients with specific impairment and/or lesion profiles. A final goal is to identify the optimal dosage of aphasia therapy. Given that chronic aphasia is associated with especially poor quality of life, even worse than conditions such as cancer [150], we propose a more progressive research agenda aimed at larger trials with personally meaningful outcomes to establish best practices for aphasia rehabilitation.