Determinants of Visceral Infarction in Acute Cardioembolic Stroke Due to Atrial Fibrillation
Article information
Dear Sir:
Atrial fibrillation (AF) is associated with an increased risk of systemic embolism and cardioembolic stroke. However, research on coexisting subdiaphragmatic visceral infarctions (SDVIs) with acute ischemic stroke due to AF is scarce. The most frequent localization of SDVI is the kidneys [1]. Acute kidney injury following acute renal infarction can occur in 20% to 40% cases, and chronic kidney injury, in about 34% of cases [2]. Splenic infarction was the second most common SDVI in AF-related acute ischemic stroke, with a 5% mortality rate [3]. Acute mesenteric ischemia is an infrequent event, though with high mortality risk [4]. SDVI diagnosis is frequently missed or delayed because of nonspecific clinical symptoms. Therefore, it is essential to predict coexisting acute SDVI in AF-related stroke patients.
Patients with acute cardioembolic stroke due to nonvalvular AF within 7 days of the onset of symptoms were prospectively included at two tertiary hospitals between April 2016 and February 2018. All subjects were examined using abdominal magnetic resonance imaging (MRI) and transthoracic echocardiography (TTE) within 7 days of onset. Detailed criteria of subjects and MRI protocols were described in the Supplementary methods [5] and Supplementary Figure 1.
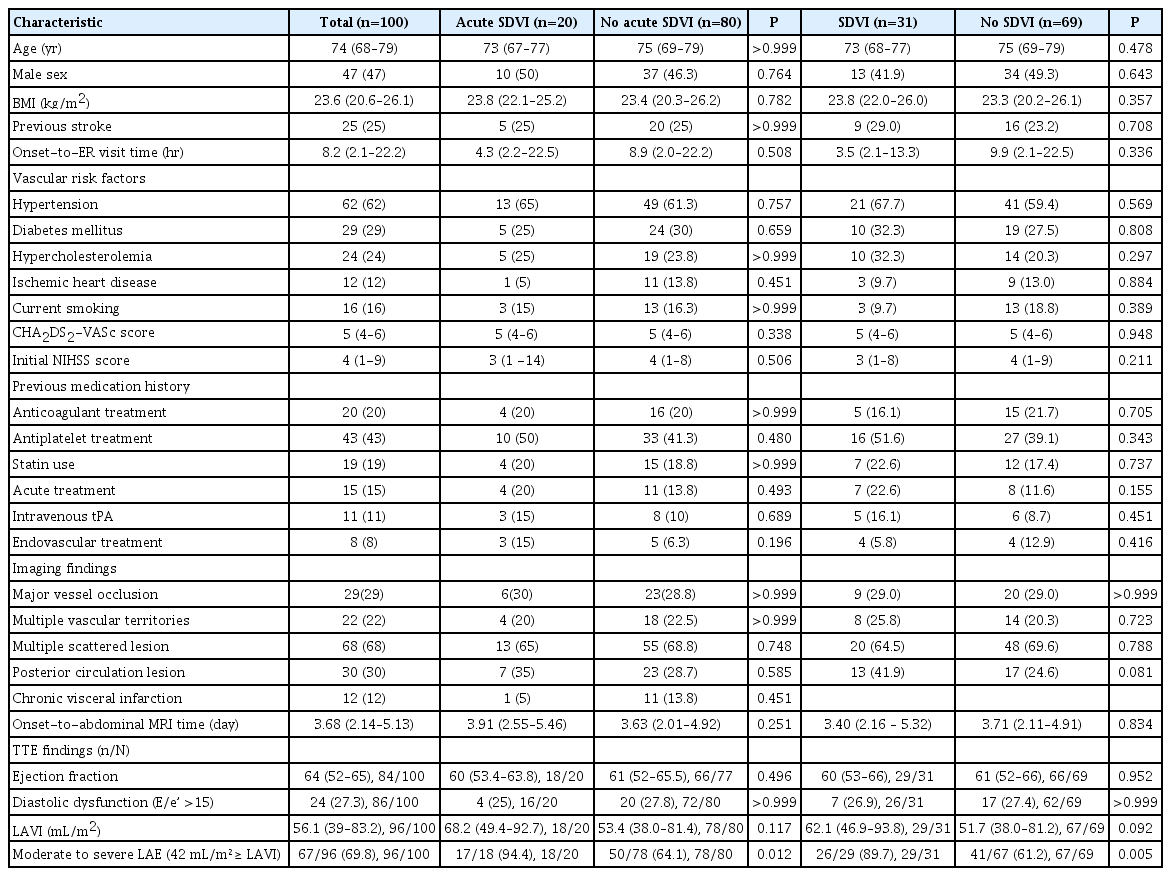
A total of 100 consecutive nonvalvular AF-related acute cardioembolic stroke patients were enrolled. The median age was 74 years (interquartile range, 68 to 79), and 47% were male. Among those patients, acute and chronic SDVIs occurred in 31 (31%) patients. Comparisons of baseline characteristics between each group are shown in Table 1. One patient had acute and chronic SDVI simultaneously. Twenty-three acute coexisting SVDIs were found in 20 patients (20%): 20 cases of renal infarction (Supplementary Figure 2), two splenic infarction, and one mesenteric infarction due to superior mesenteric artery occlusion. Of the 20 patients with acute renal infarction, four had an acute infarction in both kidneys. Furthermore, 12 patients had previous chronic SDVIs (10 with renal, two splenic infarction). One patient had chronic renal infarction on both sides. No hepatic infarction was shown (Supplementary Table 1). In 96 patients with available TTE data, the rate of moderate to severe left atrial enlargement (LAE), defined as volume/body surface area ≥42 mL/m², was 69.8%.
In the multiple logistic regression model, moderate to severe LAE was significantly associated with the coexistence of acute SDVIs in patients with acute ischemic stroke due to AF (adjusted odds ratio [aOR], 11.40; 95% confidence interval [CI], 1.29 to 100.97; P=0.03), and also a significant determinant for the presence of all SDVIs stages (aOR, 5.12; 95% CI, 1.37 to 19.15; P=0.02) (Table 2).
Our study found that 31% of patients with acute cardioembolic stroke attributed to AF, presenting within 7 days of onset, showed some stage of SVDI. Acute coexisting SVDIs occurred in 20% of total cases and chronic SDVIs in 12%. In one postmortem study, SDVI was observed in 38.6% of patients with cardioembolic stroke [1]. However, this study had a potential for selection bias (only fatal strokes were included), and it could not assess the temporal link between acute cardioembolic stroke due to AF and concomitant acute SDVIs.
Abdominal MRI, including diffusion-weighted imaging, can easily differentiate between recent and old infarctions, and we performed an abdominal MRI within 7 days of symptom onset. Furthermore, there is no additional harm to patients suspected of accompanying acute renal infarction due to no need to use contrast agents compared to abdominal computed tomography. However, there is a limit to the implementation in patients with unstable vital signs. In a small MRI-based study, four (14.8%) out of 27 stroke patients with AF showed recent infarctions and three (11.1%) old infarctions [6]. Another MRIbased study found SDVIs in 10 (21.3%) out of 47 patients with ischemic stroke due to AF [7]. Of those, 10.7% had acute and 10.6% chronic infarctions. In terms of acute SDVIs, our study showed a higher incidence than previous studies. This finding may be due to a higher rate of vascular comorbidities and a higher risk of the CHA2DS2-VASc score in our subjects. The prevalence of chronic SDVIs was similar to previous MRI-based studies.
Our results suggest that a moderate-to-severe LAE on TTE is more closely related to a greater risk of acute coexisting SDVIs, in acute cardioembolic stroke due to AF, compared to normal left atrium (LA) or mild LAE, which were independent of the SDVI stage. Atrial remodeling and impaired atrial contractility may contribute to LAE, which in turn may lead to more blood stasis and endothelial injury, thereby predisposing to thromboembolism. Thrombus formation is more likely to occur in the larger LA. Large LA volume could be a good predictor of an increased risk of ischemic stroke, systemic embolism, and mortality in AF patients without a history of previous stroke [8]. The association between LAE and recurrent stroke is already well known. However, before our study, the association between LAE and systemic embolism in AF-related acute cardioembolic stroke was less clear. Based on our results, evaluating the coexistence of acute SDVIs is critical in AF-related acute cardioembolic stroke patients with moderate-to-severe LAE.
The interpretation of our results require caution because of selection bias. In contrast to the autopsy study, some patients with unstable vital signs or malignant stroke may have been excluded from the study. Non-categorization of AF type is also considered an important limitation. However, LA volume is positively correlated with AF duration and could be a good marker of AF duration.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2020.03972.
Localization of subdiaphragmatic visceral infarction
Flow diagram of study participants. SU, stroke unit; SDVI, subdiaphragmatic visceral infarction.
Abdominal diffusion-weighted imaging (A) with the apparent diffusion coefficient (B) of 72-year-old patient, showing an area of acute renal infarction in the lower pole of the right kidney (arrows).
Notes
The authors have no financial conflicts of interest.