Etiology, 3-Month Functional Outcome and Recurrent Events in Non-Traumatic Intracerebral Hemorrhage
Article information
Abstract
Background and Purpose
Knowledge about different etiologies of non-traumatic intracerebral hemorrhage (ICH) and their outcomes is scarce.
Methods
We assessed prevalence of pre-specified ICH etiologies and their association with outcomes in consecutive ICH patients enrolled in the prospective Swiss Stroke Registry (2014 to 2019).
Results
We included 2,650 patients (mean±standard deviation age 72±14 years, 46.5% female, median National Institutes of Health Stroke Scale 8 [interquartile range, 3 to 15]). Etiology was as follows: hypertension, 1,238 (46.7%); unknown, 566 (21.4%); antithrombotic therapy, 227 (8.6%); cerebral amyloid angiopathy (CAA), 217 (8.2%); macrovascular cause, 128 (4.8%); other determined etiology, 274 patients (10.3%). At 3 months, 880 patients (33.2%) were functionally independent and 664 had died (25.1%). ICH due to hypertension had a higher odds of functional independence (adjusted odds ratio [aOR], 1.33; 95% confidence interval [CI], 1.00 to 1.77; P=0.05) and lower mortality (aOR, 0.64; 95% CI, 0.47 to 0.86; P=0.003). ICH due to antithrombotic therapy had higher mortality (aOR, 1.62; 95% CI, 1.01 to 2.61; P=0.045). Within 3 months, 4.2% of patients had cerebrovascular events. The rate of ischemic stroke was higher than that of recurrent ICH in all etiologies but CAA and unknown etiology. CAA had high odds of recurrent ICH (aOR, 3.38; 95% CI, 1.48 to 7.69; P=0.004) while the odds was lower in ICH due to hypertension (aOR, 0.42; 95% CI, 0.19 to 0.93; P=0.031).
Conclusions
Although hypertension is the leading etiology of ICH, other etiologies are frequent. One-third of ICH patients are functionally independent at 3 months. Except for patients with presumed CAA, the risk of ischemic stroke within 3 months of ICH was higher than the risk of recurrent hemorrhage.
Introduction
Non-traumatic intracerebral hemorrhage (ICH) is a devastating disease with poor functional outcome and high mortality and morbidity [1,2]. There is a significant lack of evidence-based treatment options for acute care and secondary prevention [3,4]. Treatment and prognosis of ischemic stroke improved dramatically during the last decades, which seems directly related to an improved understanding of the heterogeneity of different stroke subtypes, their clinical presentation and prognosis as depicted by widely used classifications of stroke etiology [5-7].
In contrast, no widely accepted etiological classification exists for ICH [8]. Most previous studies determined prognosis of patients with ICH according to ICH location (a surrogate for underlying small vessel disease [9,10]) or distribution of cerebral microbleeds [11] (a surrogate for cerebral amyloid angiopathy [CAA]). A meta-analysis provided valuable insights, but was based on historical data without further differentiation of subtypes [12].
ICH is caused by various underlying pathologies [13-15] and mechanistic classifications have been proposed, although with some inherent limitations. Current knowledge on the utility of a mechanistic classification of ICH subtypes is limited to small and/or single center studies with few outcomes (i.e. mortality) and limited follow-up periods [8].
The aim of our study was to determine the frequency of different etiologies of ICH using a mechanistic ICH classification and their association with 3-month outcomes (functional outcome, recurrent ICH, and ischemic stroke) in a large, national dataset of ICH patients admitted to Stroke Units and Stroke Centers in Switzerland.
Methods
Study design and patient selection
We analyzed data of consecutive patients with non-traumatic ICH enrolled in the prospective, compulsory Swiss Stroke Registry (SSR, 2014-2019). The SSR is a national registry designed for quality control and research in stroke. All consecutive adult patients with cerebrovascular events (transient ischemic attack, ischemic stroke, and ICH) who are treated in one of the 10 Swiss Stroke Centers (tertiary referral centers offering the entire spectrum of stroke care with 24/7 access to magnetic resonance imaging [MRI] and interventional neuroradiology) and 13 Stroke Units (certified according to Swiss Stroke Unit and Stroke Center criteria, equivalent to European Stroke Organisation Certification committee recommendations [16]) are enrolled in the SSR. We included all patients aged >18 years with imaging proven diagnosis of ICH. We excluded patients with missing information on ICH etiology.
Baseline data and data collection
Local investigators at the participating SSR centers recorded standardized and prespecified variables using electronic case report forms. This procedure has been described previously and the dataset has been used in prior research [17,18]. The encoded, web-based database is hosted by the Clinical Trials Unit Basel. We extracted the following variables from the prospective databank: (1) demographics: age, sex; (2) risk factors and comorbidities: pre-existing hypertension, diabetes, hyperlipidemia, atrial fibrillation, smoking, intake of antiplatelets or anticoagulants prior to the event, type of oral anticoagulants (vitamin K antagonists or direct oral anticoagulants), history of previous ischemic stroke, transient ischemic attack and ICH; (3) clinical presentation on admission: National Institutes of Health Stroke Scale (NIHSS), Glasgow coma scale (GCS), systolic and diastolic blood pressure, time from symptom onset to admission; and (4) management: treatment in a stroke center or stroke unit, discharge destination. Data regarding ICH etiology and outcomes were also extracted from the prospective databank (see below). We applied a plausibility check and set implausible values to missing.
Etiology of ICH
ICH etiology was prospectively determined by local investigators according to six prespecified, mutually exclusive categories based on an adapted version of the mechanistic SMASH-U [13] classification (Figure 1). The original SMASH-U classification used a hierarchical approach as follows: structural lesion > systemic disease > medication > amyloid angiopathy > hypertension > unknown.
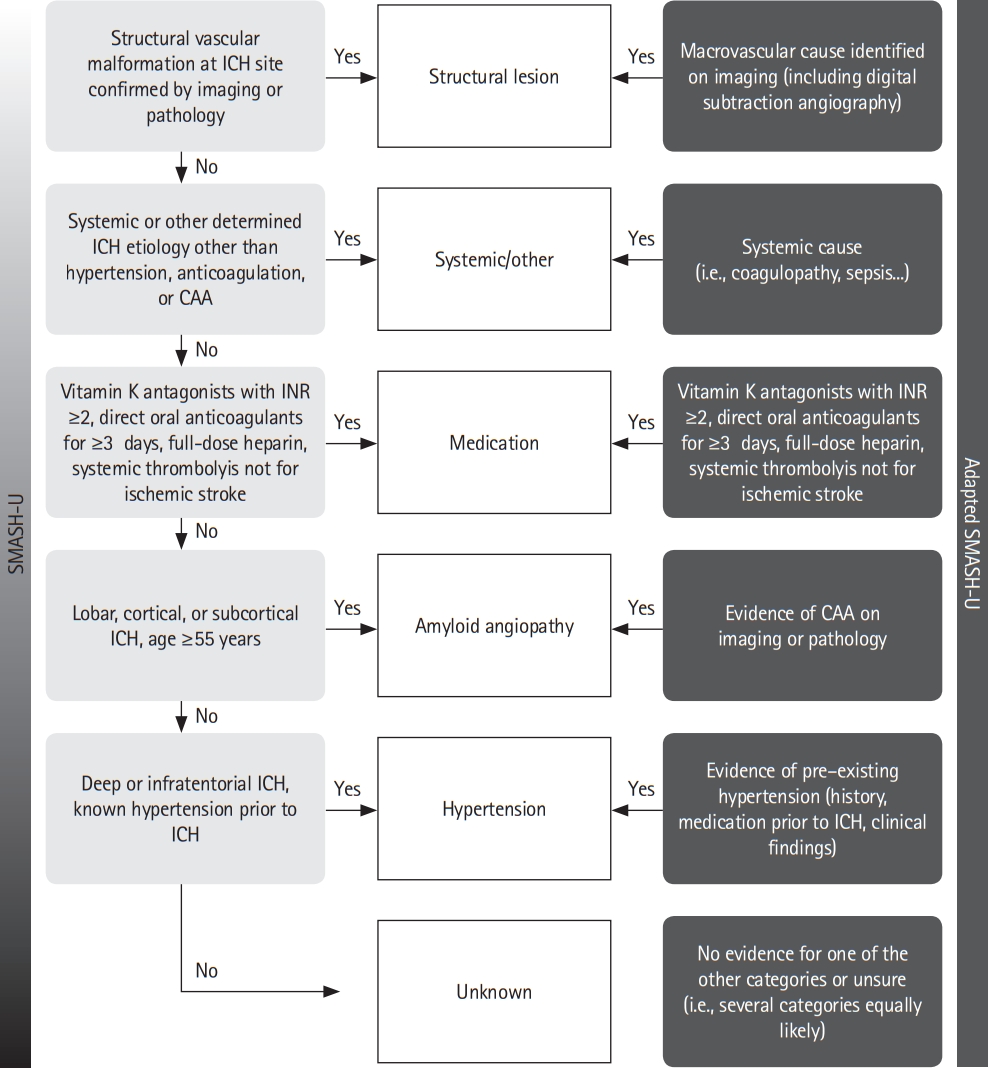
Mechanistic classification of intracerebral hemorrhage (ICH) etiology: comparison of the original and adapted SMASH-U (structural lesion > systemic disease > medication > amyloid angiopathy > hypertension > unknown) classifications. CAA, cerebral amyloid angiopathy; INR, international normalized ratio.
For the purpose of the SSR, we did not mandate a strict hierarchy as in the original SMASH-U classification but the etiology, which was most likely causal for ICH as judged by the local investigator based on all available evidence from clinical and radiological exams. Computed tomography and magnetic resonance angiography are standard of care in Swiss Stroke Units and Centers to detect vascular sources of bleeding. As MRI is frequently used in Switzerland, diagnosis of CAA was mostly based on MRI, medical history (prior lobar ICH) or using the Edinburgh-CAA criteria [19], once they were published.
Outcomes
All patients enrolled in the SSR received standardized follow-up assessments by local investigators at 3 months during either outpatient visits or structured telephone interviews. If a patient and/or their next-of-kin could not be contacted, investigators contacted general practitioners and collected medical reports of other acute care hospitals and rehabilitation facilities. The following outcomes were assessed: functional outcome using the modified Rankin Scale (mRS), recurrent ICH and ischemic stroke within 3 months after the index event. For this analysis, we defined functional independence as mRS 0 to 2.
Statistical analysis
Statistical analysis was performed using Stata version 15.0 (StataCorp., College Station, TX, USA). We presented absolute and relative count (%) for categorical variables, mean and standard deviation for normally distributed, continuous variables and median and interquartile range (IQR) for non-normally distributed variables. We compared groups using chisquare test or Fisher’s exact test for categorical variables, as well as analysis of variance (ANOVA) or Kruskal-Wallis test for continuous variables. Patients with missing values (including lost to follow-up) were excluded from regression analyses (listwise deletion). We calculated odds ratios (ORs) with corresponding 95% confidence intervals (CIs) comparing the respective etiologic subgroup to a subgroup consisting of all other etiologies. For the multivariable regression models, we defined covariates prior to statistical analysis based on literature and pathophysiologically plausible differences. Observed differences were considered significant if P<0.05.
First, we determined the frequency of different ICH etiologies within the entire cohort and assessed association with key clinical characteristics at baseline (NIHSS, GCS, systolic blood pressure, and onset to admission time) using uni- and multivariable linear regressions. We adjusted for the following parameters: age, antiplatelet therapy prior to ICH onset, anticoagulation prior to ICH onset, sex, hypertension, diabetes, hyperlipidemia, smoking, atrial fibrillation history of stroke, and history of ICH.
Second, we determined the frequency of functional independence (defined as mRS 0 to 2) and mortality at 3 months and assessed the association with ICH etiology using univariable and multivariable logistic regressions comparing the respective etiologic subgroup to a subgroup consisting of all other etiologies.
Third, we determined the rate of recurrent ICH and ischemic stroke within 3 months and assessed association with ICH etiology using uni- and multivariable logistic regressions. Regression models on functional independence and recurrent cerebrovascular events were adjusted for the following predefined parameters: age, sex, treatment at a stroke center (compared to treatment at a stroke unit), NIHSS, GCS, systolic and diastolic blood pressure on admission, onset-to-admission time, antiplatelet and anticoagulation therapy prior to ICH onset, history of ischemic stroke or ICH, hypertension, diabetes, hyperlipidemia, smoking, and atrial fibrillation.
In a subset of patients, we tested the interrater reliability for ICH etiology using Gwet’s AC method (Stata package kappaetc), which has been used in previous publications in national stroke databases [20]. We performed a sensitivity analysis restricting the dataset to patients without antithrombotic therapy. Initial etiology adjudication was performed by the local treating physicians. Second rating was performed by experienced research fellows (A.M. and A.A.P.) based on the available clinical information.
Standard protocol approvals, registrations, and patient consents
This project was approved by the competent ethical board in Bern (Project-ID 2019-00689). As to Swiss legislation (law on highly specialized medicine; “Hochspezialisierte Medizin” HSM; Art. 39 Abs. 2bis KVG) and ethics approval, all patients treated in one of the certified Swiss Stroke Units or Stroke Centers are enrolled in the SSR database. Patients who refused data use for scientific purposes were excluded from this analysis.
Data availability
Anonymized data will be shared upon reasonable request from any qualified researcher after clearance by the SSR steering committee and the competent ethics committee.
Results
Study population
Among 2,926 patients with ICH enrolled in the SSR between 2014 and June 2019, 2,650 patients (90.6%) had available information on ICH etiology and were included (Supplementary Figure 1). Mean age in our population was 72 years, 46.5% of patients were female, the median admission NIHSS and GCS were 8 (IQR, 3 to 15) and 15 (IQR, 12 to 15), respectively. The median onset to admission time was 3.8 hours (IQR, 1.4 to 13.3), brain MRI was performed in 42.5% of the patients, and 77.4% of the patients were treated at a stroke center. Baseline characteristics of the entire cohort and according to ICH etiologies are displayed in Table 1. In a random subset of 59 patients (2.4%), interrater reliability was moderate (Gwet’s AC1, 0.49; 95% CI, 0.30 to 0.68). When limited to patients without antithrombotic therapy (44 patients, 1.7%), the interrater reliability was substantial (Gwet’s AC1, 0.61; 95% CI, 0.36 to 0.83).
Frequency of ICH etiologies
Among the 2,650 ICH patients, 1,238 (46.7%) patients had ICH due to hypertension, 227 (8.6%) had ICH due to antithrombotic therapy, 217 patients (8.2%) had ICH due to CAA, 128 (4.8%) patients had ICH due to a macrovascular cause, 274 (10.3%) patients had ICH due to other determined etiology, and in 566 (21.4%) patients the etiology of ICH was unknown (Figure 2).
In patients taking antithrombotic therapy, ICH was classified as ICH due to antithrombotic therapy in 46 (6.3%) patients taking antiplatelet agents and 212 (37.3%) patients taking anticoagulation with 31 patients taking both anticoagulation and antiplatelet agents (13.7% of all patients with ICH due to antithrombotic therapy). Of 2,053 patients (79.9%) with a diagnosis of arterial hypertension, in only 1,238 (60.3%) ICH was classified as to be due to hypertension.
Distribution of age, baseline NIHSS, systolic blood pressure on admission, and onset-to-admission-time among different ICH etiologies are displayed in Figure 3. In multivariable linear regression analysis, we found that ICH due to hypertension was independently associated with higher NIHSS on admission (2 points in the adjusted analysis), higher systolic blood pressure (19 mm Hg), and lower onset-to-admission time (–7 hours and 25 minutes). Antithrombotic therapy-associated ICH and ICH of unknown etiology were independently associated with lower admission blood pressure (–6 mm Hg, each). CAA was independently associated with less neurological impairment (NIHSS –3 points, GCS +1 point, both P<0.001), lower systolic blood pressure (–10 mm Hg, P<0.001), and longer onset-to-admission delay (7 hours and 59 minutes, P<0.001). Patients with ICH due to another determined etiology presented with a significantly lower NIHSS (–3 points, P<0.001), higher GCS (1 point, P=0.003), lower systolic blood pressure (–9 mm Hg, P<0.001) and had a longer onset-to-admission delay (10 hours and 46 minutes, P<0.001).
Functional outcome
Functional outcome (mRS score) at 3 months was available for 2,250 patients (84.9%). Patients with missing functional outcomes had more favorable baseline characteristics (e.g., lower NIHSS, higher GCS, lower prevalence of prior anticoagulant therapy) than those with available outcomes (Supplementary Table 1). At 3 months, 880 patients (39.1% of all 2,250 patients with available mRS) had achieved functional independence (Figure 4). We observed the highest rate (49.1%) of patients with functional independence among those with ICH due to other determined etiology and the lowest (22.8%) among patients with ICH due to antithrombotic therapy. ICH due to hypertension was independently associated with functional independence (adjusted odds ratio [aOR], 1.33; 95% CI, 1.00 to 1.77; P=0.05) (Table 2). For patients with ICH due to antithrombotic therapy, we found decreased odds for functional independence in the univariable regression (OR, 0.43; 95% CI, 0.30 to 0.60; P<0.001), but this finding was attenuated by adjustment for potential confounders (aOR, 0.70; 95% CI, 0.41 to 1.19; P=0.191). Missing data relevant for multivariable analysis were age (8.0%), onset to admission time (8.0%), and NIHSS (5.8%).
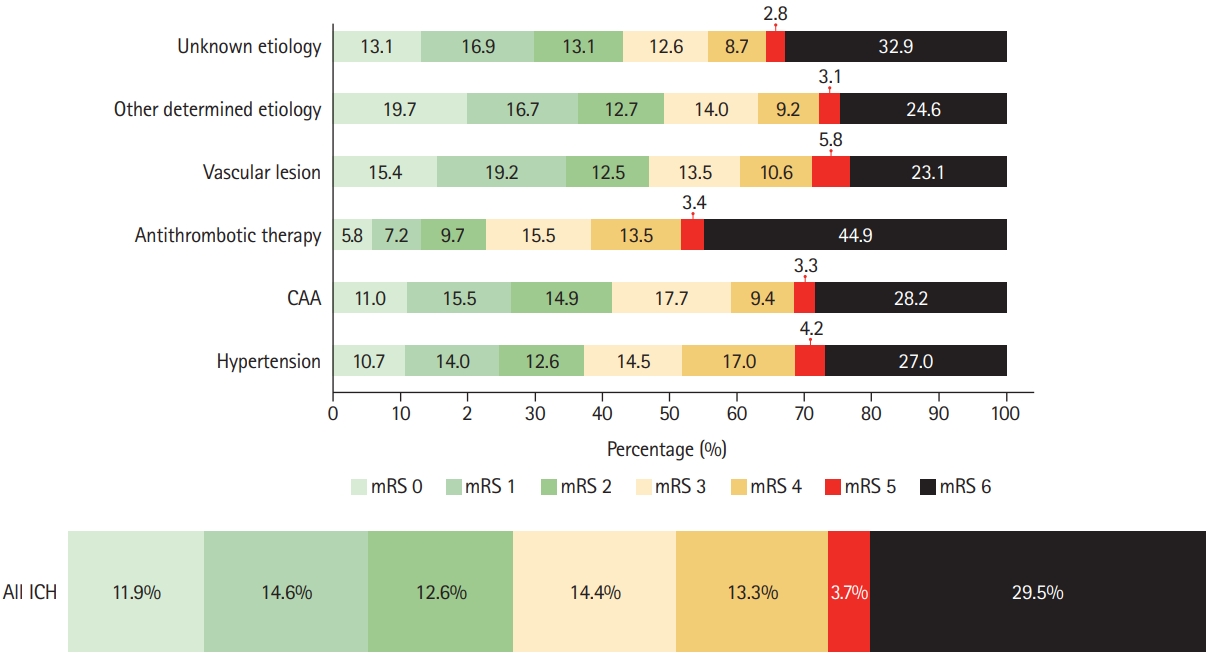
Functional outcomes at 3 months according to intracerebral hemorrhage (ICH) etiology. CAA, cerebral amyloid angiopathy; mRS, modified Rankin Scale.
Recurrent ICH and stroke
Outcome data on recurrent ICH and ischemic stroke were available for 2,299 patients (86.8%). During the follow-up period, we observed 98 events in 97 patients (event rate 4.2%) (Figure 5): 47 (2.1%) recurrent ICH and 51 (2.3%) ischemic strokes. In patients with CAA and unknown ICH etiology, the rate of recurrent ICH was higher than the risk of ischemic stroke. For all other etiologies, the risk of ischemic stroke was higher than the risk of recurrent ICH. In multivariable analysis, CAA was independently associated with a higher risk of recurrent ICH within 3 months (aOR, 3.38; 95% CI, 1.48 to 7.69; P=0.004) (Table 3) and patients with ICH due to hypertension had a significantly lower risk of recurrent ICH (aOR, 0.42; 95% CI, 0.19 to 0.93; P=0.031).

All cerebrovascular events, Ischemic stroke and recurrent intracerebral hemorrhage (ICH) at 3 months according to ICH etiology. CAA, cerebral amyloid angiopathy.
Discussion
This study has the following main findings. (1) One out of two ICH is due to hypertension, but other etiologies are frequent with one out of five patients having unknown ICH etiology. (2) Arterial hypertension and use of antithrombotic therapy prior to ICH onset are frequent among patients with ICH, but the link between these population risk factors and attributable etiology of ICH remains unclear. (3) One out of three patients with ICH was functionally independent after 3 months. (4) Cerebrovascular events within the first 3 months after ICH are frequent (4.2%) with diverging absolute and relative risks according to ICH etiology. (5) The rate of ischemic stroke was higher than that of recurrent ICH in all etiologies but CAA and ICH of unknown etiology.
Our study provides novel and unique data. We are the first to report (1) functional outcome and rates of (2) recurrent ICH and (3) ischemic stroke at 3 months associated with the SMASH-U classification. Furthermore, our study is 2.5 times larger than the largest available dataset [21] and comprises a national, multi-center dataset overcoming many limitations from previous, smaller, single center studies or other approaches to classify ICH as for example using hematoma location [9,10]. The findings of our study expand and refine current knowledge on ICH etiology. This is the first analysis of a national dataset presenting prospectively collected data on the frequency of different ICH etiologies. It is also the first study to assess the association of different ICH etiologies with functional outcome and occurrence of ischemic stroke and recurrent ICH.
Our results expand on prior findings as we found that etiologies other than hypertension [13,15,22] are frequent and make up more than half of non-traumatic ICH cases. Compared to the original study of the SMASH-U classification [13], we found higher frequency of ICH due to other determined cause, lower frequency of CAA and lower causal attribution of antithrombotics as etiology of ICH. This finding might reflect temporal changes (the original SMASH-U study was published nearly 10 years ago) of etiological work-up including MRI in 43% of patients in our study. The high use of MRI might have helped to ruleout CAA in cases of lobar ICH (classified as CAA in SMASH-U) and reveal other determined etiologies including posterior reversible encephalopathy syndrome, reversible cerebral vasoconstriction syndrome or tumor. The difference in ICH due to antithrombotic therapy is not explained by differences in the frequency of prior anticoagulation therapy, which was higher in our study (22% vs. 13%). This finding may probably reflect major differences in considering anticoagulation not as the underlying cause of ICH but rather a complicating covariate [23,24]. Actually, many patients with prior anticoagulation therapy were classified as ICH due to hypertension in our study. Despite different workup paths, the frequency of ICH with unknown cause was comparable between both studies with about 20%.
The findings of our study provide major new insights as we provide novel information on functional outcome 3 months after ICH of a national cohort 10 times larger than any prior etiological studies [25,26]. This is the first study to show a high rate of functionally independent patients 3 months after ICH in a national dataset of patients hospitalized for ICH. The rate of good outcome in our study was higher than that observed in recent randomized controlled trials [21,27,28] but in line with recent observational data of patients receiving maximal care for ICH [29]. In addition, almost three-fifths of ICH survivors in our study achieved mRS ≤2 and more than three-fourths of survivors had mRS ≤3 after 3 months, clearly refuting widespread pessimism about outcome of ICH patients [30]. We urge caution in the interpretation of our findings on functional outcome as there was a number of missing data and we did not have information on hematoma volume and location, major determinators of outcome. However, we included stroke severity (NIHSS and GCS) in the models and patients with missing outcomes had lower NIHSS, less often anticoagulation and were more often discharged home. Therefore, it is likely that we rather under- than overestimated functional independence.
Our study is the first to report rates of ischemic stroke and recurrent ICH according to different etiologies of ICH in a large, national cohort study. Prior studies used either hematoma location [9,10] (as surrogate marker) or focused on selected subgroups like CAA [11,12]. We found that the risk of ischemic stroke is higher than that of recurrent ICH in all but two etiologies: ICH due to CAA and ICH of unknown etiology. The risk of recurrent ICH in our study was higher than that of previous studies in patients with CAA [11].
This study has several strengths. (1) This study provides a comprehensive overview about the frequency of different ICH etiologies assessing association with functional outcome, recurrent ICH and ischemic stroke. (2) We used data from a large national dataset that prospectively enrolled all consecutive patients from certified Swiss stroke units and stroke centers. The dataset comprised data from academic, non-academic and regional hospitals providing stroke care for the entire Swiss population. This is an argument against selection bias and for generalizability of our findings. (3) Patients received diagnostic work-up according to current standards and the rate of MRI was high (42%). (4) Despite the size of our study involving 23 centers over 4 years, we achieved a high rate of follow-up completeness (84.9% for functional outcomes 86.8% for cerebrovascular events) comparable to similar studies [31].
Our study has the following limitations. (1) This was a retrospective analysis of prospectively collected data, which bears a risk of bias. (2) ICH etiology was determined by local investigators without central adjudication, which is a source of bias. However, this reflects real world practice and could also serve as argument for generalizability of our findings. (3) Prior anticoagulation was highly prevalent in our study cohort but anticoagulation therapy was judged causal for the ICH only in a minority of patients. Whether anticoagulation should be seen as causal for ICH or as a complicating, negative prognostic factor, is debated [24] and beyond the scope of our study. (4) Although our follow-up rate was relatively high, there were still 15% of missing outcomes (mRS) and we therefore urge caution in the interpretation of our results. (5) We adjusted outcome analysis for multiple important covariates (i.e., comorbidities, GCS, NIHSS) but several important variables were not available in our dataset including hematoma location, volume and care level (do-not-resuscitate orders). (6) Inter-rater reliability of the present classification was only moderate. However, it improved to substantial when only applied to patients without antithrombotic therapy. (7) Despite the limitations inherent to the classification used in our study as outlined above, developing a novel, improved classification was beyond the scope of our study. Therefore, we urge caution in the interpretation of our findings and future studies will need to address these shortcomings.
Conclusions
Etiologic subtypes of ICH differ regarding clinical presentation and prognosis with the absolute and relative risks of recurrent ICH and ischemic stroke diverging among different etiologies. Although hypertension is the leading etiology of ICH, other causes are frequent. One-third of ICH patients are functionally independent at 3 months. These findings have direct clinical implications as they help to inform treating physicians, patients and caregivers about prognosis and may help to set priorities for secondary prevention strategies. However, a mechanistic classification of ICH etiology bears some inherent limitations and future research should focus on reliable, reproducible, and objective classification systems.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2021.01823.
Comparison of baseline characteristics for patients with available vs. missing outcomes
Study flowchart. mRS, modified Rankin Scale.
Notes
Disclosure
Martina B. Goeldlin: grants from SAMW/Bangerter-Rhyner-Foundation (YTCR 13/18), Stroke Society, Mittelbauvereinigung at University of Bern. Congress support from Pfizer (outside the submitted work); Davide Strambo: research grants from Swiss Heart Foundation and University of Lausanne, congress support from Bristol Myers Squibb. All fees are paid to his institution; Carlo W. Cereda: advisory board for Bayer, Ischemaview, Astra Zeneca, Medtronic, Portola/Alexion; Bastian Volbers: personal fees from Pfizer AG/Bristol-Myers Squibb SA, personal fees from Bayer AG, grants from Institutional grant (Inselspital), personal fees from Ipsen Pharma, personal fees from CSL Behring, outside the submitted work; Leo H. Bonati: grants from the Swiss National Science Foundation, the University of Basel, the Swiss Heart Foundation, and the “Stiftung zur Förderung der gastroenterologischen und allgemeinen klinischen Forschung sowie der medizinischen Bildauswertung”; an unrestricted research grant from AstraZeneca; and consultancy or advisory board fees or speaker’s honoraria from Amgen, Bayer, Bristol-Myers Squibb, Claret Medical, and InnovHeart, and travel grants from AstraZeneca and Bayer; David J. Seiffge: advisory board for Bayer and Portola/Alexion, Research grant from the Bangerter-Rhyner Foundation. All other authors have nothing to disclose
Acknowledgements
We thank Mattia Branca for the statistical consulting. We thank all SSR investigators as listed in the online supplement, who have contributed in a significant manner to the present manuscript.
The following collaborators participated in the Swiss Stroke Registry and contributed in a significant and documentable manner but do not qualify as regular co-authors to the manuscript “Etiology of non-traumatic intracerebral hemorrhage and association with functional outcome and recurrent events - data from the Swiss Stroke Registry”. They have confirmed that their names can be listed in any manuscript (i.e. appendix, acknowledgement) arising from this registry.