Dear Sir:
Spontaneous intracerebral hemorrhage (ICH) accounts for approximately 20% of all strokes and is a leading cause of mortality and morbidity worldwide [
1]. Despite advances in medical research, the treatment for ICH remains strictly supportive [
2,
3]. Efforts are ongoing to develop new targets for improving outcomes after ICH [
4].
In-hospital neurological deterioration affects approximately 10% to 30% of the patients with ICH [
5-
7], which includes early and delayed neurological deterioration. Thus, preventing in-hospital neurological deterioration after ICH is a logical step and represents a promising approach to improve outcomes after ICH. Currently, no valid risk model is available to identify high-risk populations for neurological deterioration after ICH in routine clinical practice or clinical trials. In this study, we aimed to develop a risk score (ICH progression score) to predict in-hospital neurological deterioration after ICH using routinely collected variables at presentation.
The derivation and internal validation cohorts were obtained from the Beijing Registration of Intracerebral Hemorrhage [
8]. External validation was based on the China National Stroke Registry [
9] and the in-hospital medical complications after acute stroke (iMCAS) study [
10].
In this study, in-hospital neurological deterioration after ICH was defined as an episode in which a patient experienced a persistent increase in National Institutes of Health Stroke Scale score ≥4, a decline in Glasgow Coma Scale (GCS) score ≥2, or death during hospitalization.
The baseline characteristics of the derivation cohort and the internal and external validation cohorts are presented in
Table 1. Univariate and multivariate analyses for predictors of in-hospital neurological deterioration after ICH in the derivation cohort are shown in
Supplementary Tables 1 and
2. To derive an integer value for each predictor, the β coefficients were multiplied by four and rounded to the closest integer. Finally, age, sex, medical history of diabetes mellitus and atrial fibrillation, GCS score, dysphagia, hematoma location, hamartoma volume, and blood glucose level were included in the ICH progression score. The ICH progression scores ranged from 0 to 32 (
Table 2). The five-level risk categories were assigned in six-point increments. The rate of in-hospital neurological deterioration increased steadily with increasing ICH progression scores (
Figure 1).
The predictive performance (area under the receiver operating characteristic curve [AUROC]) of the ICH progression score in the derivation (n=1,309) and internal validation cohorts (n=655) was 0.840 (95% confidence interval [CI], 0.813 to 0.867) and 0.845 (95% CI, 0.808 to 0.881) (
Supplementary Table 3). The predicted and observed risks of in-hospital neurological deterioration after ICH were in close agreement according to the 10 deciles of predicted risk in the derivation (r=0.96,
P<0.001) and internal validation (r=0.95,
P<0.001) cohorts (
Supplementary Figure 1A and
B). In external validation cohort-1 (n=3,255) and -2 (n=314), the ICH progression score showed good discrimination with an AUROC of 0.810 (95% CI, 0.789 to 0.832) and 0.831 (95% CI, 0.696 to 0.966) (
Supplementary Table 3). The plot of observed versus the predicted risk of in-hospital neurological deterioration after ICH showed a high correlation between observed and predicted risk in the external validation cohort-1 (r=0.93,
P<0.001) and -2 (r=0.91,
P<0.001) (
Supplementary Figure 1C and
D). The Hosmer-Lemeshow test was not significant in the tested cohorts (all P>0.05). The Snell R-square and Nagelkerke R-square values of the Hosmer-Lemeshow goodness-of-fit test are shown in
Supplementary Table 4. In the sensitivity analysis, the ICH progression score showed similar good discrimination in several subgroups of patients with different clinical characteristics (AUROC range, 0.772 to 0.883) (
Supplementary Table 5).
To the best of our knowledge, this is the first study to develop a risk score to predict in-hospital neurological deterioration after ICH. The ICH progression score is unique as it was derived from a large, multicenter, and prospective ICH cohort, which included consecutive patients with ICH, was outside of clinical trials, and was more reflective of real-world clinical practice. Additionally, the ICH progression score consists of factors that are readily available at the presentation. Using a simple score, it can easily be applied in clinical practice or clinical trials.
The predictive performance of the ICH progression score was shown to be accurate in risk stratification and outcome prediction in the derivation, internal, and external validation cohorts (AUROC range, 0.810 to 0.845), respectively. In addition, in the sensitivity analysis, the ICH progression score was valid in several prespecified subgroups of patients with different clinical characteristics.
In-hospital neurological deterioration, whether early or late, was significantly associated with short- and long-term death, poor functional outcome, cognition, and quality of life after ICH [
5-
7]. Using the ICH progression score, clinicians can identify patients at high risk of developing in-hospital neurological deterioration after ICH. Early prediction of in-hospital neurological deterioration after ICH would help identify vulnerable patients and implement tailored preventive strategies. In addition, it could be used as a selection criterion in nonrandomized studies to control for case-mix variation and in controlled studies. The potential etiology of in-hospital neurological deterioration after ICH might be heterogeneous and dynamically changing. For example, at the early stage after ICH (e.g., within 24 hours after onset), hematoma expansion, intraventricular hemorrhage, and rapidly increased intracranial pressure might be potential causes of neurological deterioration, and at the later stage after ICH (e.g., 24 hours to 14 days after onset), pre-hematoma edema, hydrocephalus, infection, and other medical complications might cause the condition of ICH patients to worsen. Based on the potential risk and etiology of in-hospital neurological deterioration after ICH, clinicians should apply tailored preventive and treatment strategies.
Our study had some limitations. First, we cannot rule out the possibility that additional baseline variables (unmeasured confounders) might have an impact on the risk of in-hospital neurological deterioration after ICH. Second, our study included only hospitalized patients, and patients who died in the emergency department or were treated in outpatient clinics were not included. Finally, both the derivation and validation cohorts were derived from the Asian population.
In summary, the ICH progression score is a valid clinical grading scale for predicting in-hospital neurological deterioration after ICH at presentation and would be a useful tool for personalized care and clinical trials in the prevention of in-hospital neurological deterioration after ICH.
The study protocol was approved by the Institutional Review Board (IRB) of the Beijing Tiantan Hospital (KY2014-023-02). Written informed consent from patients or their legal representatives.
Supplementary materials
Supplementary Table 1.
Univariable predictor of in-hospital neurological deterioration after ICH in the derivation cohort (n=1,309)
Supplementary Table 2.
Multivariable predictors of in-hospital neurological deterioration after ICH in the derivation cohort (n=1,309)
Supplementary Table 3.
Predictive performance of ICH progression score with regard to in-hospital neurological deterioration after ICH
Supplementary Table 4.
Calibration of the ICH progression score with regard to in-hospital neurological deterioration after ICH
Supplementary Table 5.
Sensitivity analysis of ICH progression score in the Beijing Registration of Intracerebral Hemorrhage (n=1,964)
Supplementary Figure 1.
Plot of observed versus predicted risk of neurological deterioration after intracerebral hemorrhage (ICH) in the derivation and validation cohorts. Plot of observed versus predicted risk of in-hospital neurological deterioration after ICH in the derivation, internal, and external validation cohorts according to 10 deciles of predicted risk. Overall, there was a very high correlation between the observed and predicted risks in the derivation cohort (A) (n=1,309; r=0.96; P<0.001), internal validation cohort (B) (n=655; r=0.95; P<0.001), external validation cohort-1 (C) (n=3,255; r=0.93, P<0.001), and external validation cohort-2 (D) (n=314; r=0.91, P<0.001), which indicated excellent calibration.
Acknowledgments
This study was sponsored by the Capital Health Research and Development of Special (2011-2004-03) and the Beijing Municipal Science & Technology Commission (Z131107002213009). This study was partially supported by the Nova Program of the Beijing Science and Technology Commission (2008B30), National Natural Science Foundation of China (81471208, 81641162), Beijing High-level Healthy Human Resource Project (014-3-033), and Shandong Province Key Innovation Project (2019JZZY020901).
Figure 1.
In-hospital neurological deterioration after intracerebral hemorrhage (ICH) according to the ICH progression score. The figure shows that the proportion of in-hospital neurological deterioration after ICH increased steadily with higher ICH progression scores in the derivation (n=1,309), internal validation (n=655), and two external valuation cohorts (n=3,255 and n=314).
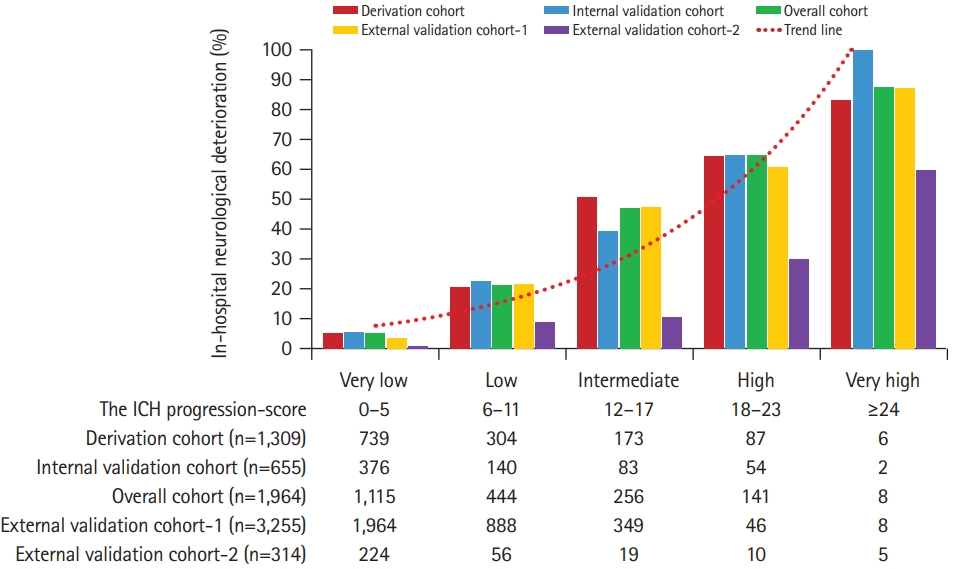
Table 1.
|
Characteristic |
Overall cohort (n=1,964) |
Derivation cohort (n=1,309) |
Internal validation cohort (n=655) |
P*
|
External validation cohort-1 (n=3,255) |
External validation cohort-2 (n=314) |
|
Age (yr) |
56.8±14.4 |
56.8±14.6 |
56.9±13.9 |
0.19 |
62.1±13.1 |
54.7±14.2 |
|
Male sex |
1,327 (67.6) |
866 (67.7) |
441 (67.3) |
0.87 |
1,995 (61.3) |
221 (70.4) |
|
Onset to hospital (hr) |
4.0 (1.90-11.0) |
4.0 (1.92-11.0) |
3.9 (1.97-11.0) |
0.76 |
10.0 (2.41-29.3) |
78 (24-96) |
|
Risk factors |
|
|
|
|
|
|
|
|
Hypertension |
1,367 (69.6) |
908 (69.4) |
459 (70.1) |
0.75 |
2,210 (67.9) |
208 (66.9) |
|
Diabetes mellitus |
289 (14.7) |
196 (15.0) |
93 (14.2) |
0.65 |
290 (8.9) |
41 (13.1) |
|
Dyslipidemia |
184 (9.4) |
109 (8.3) |
75 (11.5) |
0.03 |
230 (7.1) |
36 (11.5) |
|
Atrial fibrillation |
30 (1.5) |
20 (1.5) |
10 (1.5) |
0.99 |
54 (1.7) |
10 (3.2) |
|
History of stroke/TIA |
309 (15.7) |
208 (15.9) |
101 (15.4) |
0.79 |
889 (27.3) |
48 (15.3) |
|
Myocardial infarction |
38 (1.9) |
20 (1.5) |
18 (2.7) |
0.06 |
204 (6.3) |
26 (8.3) |
|
Heart failure |
8 (0.4) |
6 (0.5) |
2 (0.3) |
0.62 |
19 (0.6) |
3 (1.0) |
|
Current smoker |
628 (32.0) |
403 (30.8) |
225 (34.4) |
0.11 |
1,228 (37.7) |
120 (38.2) |
|
Alcohol consumption |
716 (36.5) |
470 (35.9) |
246 (37.6) |
0.47 |
367 (11.3) |
166 (52) |
|
Pre-admission anticoagulation |
21 (1.1) |
14 (1.1) |
7 (1.1) |
0.99 |
32 (1.0) |
5 (1.6) |
|
Pre-admission antiplatelet |
277 (14.1) |
181 (13.8) |
96 (14.7) |
0.62 |
291 (8.9) |
25 (7.9) |
|
Pre-stroke mRS score |
0 (0-0) |
0 (0-0) |
0 (0-0) |
0.36 |
0 (0-0) |
0 (0-0) |
|
Admission NIHSS score |
11 (3-21) |
11 (3-21) |
11 (4-21) |
0.89 |
9 (3-16) |
4 (1-10) |
|
Admission GCS score |
14 (8-15) |
14 (8-15) |
14 (9-15) |
0.26 |
14 (9-15) |
15 (14-15) |
|
Admission dysphagia |
666 (33.9) |
441 (33.7) |
225 (34.4) |
0.77 |
220 (6.8) |
24 (7.6) |
|
Admission SBP (mm Hg) |
165 (147-186) |
164 (146-186) |
167 (150-187) |
0.10 |
160 (147-180) |
158 (140-171) |
|
Admission DBP (mm Hg) |
96 (82-109) |
95 (81-108) |
98 (84-110) |
0.10 |
95 (87-106) |
93 (83-104) |
|
Hematoma location |
|
|
|
0.91 |
|
|
|
Supratentorial ICH |
1,752 (89.2) |
1,167 (89.2) |
585 (89.3) |
|
2,862 (87.9) |
282 (89.8) |
|
Infratentorial ICH |
212 (10.8) |
142 (10.8) |
70 (10.7) |
|
393 (12.1) |
32 (10.2) |
|
Hematoma volume (cm3) |
15.8 (6.0-38.6) |
15.5 (5.9-37.0) |
16.7 (6.6-40.0) |
0.20 |
12.6 (5.5-28.0) |
15 (10-30) |
|
Intraventricular extension |
655 (33.4) |
430 (32.8) |
225 (34.4) |
0.51 |
962 (29.6) |
109 (34.7) |
|
Subarachnoid extension |
264 (13.4) |
182 (13.9) |
82 (12.5) |
0.39 |
190 (5.8) |
30 (9.6) |
|
Admission WBC (109/L) |
9.79 (7.35-13.0) |
9.68 (7.29-12.9) |
10.0 (7.56-13.0) |
0.26 |
8.7 (6.7-11.3) |
8.83 (7.34-11.0) |
|
Admission glucose (mmol/L) |
7.31 (6.08-9.20) |
7.26 (6.05-9.10) |
7.49 (6.13-9.40) |
0.20 |
6.3 (5.7-7.5) |
5.04 (4.37-6.07) |
|
Admission creatinine (μmol/L) |
63.4 (52.7-77.0) |
63.1 (52.3-76.6) |
63.9 (53.8-77.0) |
0.17 |
77.0 (62.0-92.0) |
61.7 (52.1-72.1) |
|
Etiology diagnosis |
|
|
|
0.86 |
|
|
|
Primary ICH |
1,785 (90.9) |
1,193 (91.1) |
592 (90.4) |
|
- |
277 (88.2) |
|
Secondary ICH |
159 (8.1) |
103 (7.3) |
56 (8.5) |
|
- |
34 (10.8) |
|
Primary IVH |
20 (1.0) |
13 (1.0) |
7 (1.1) |
|
- |
… |
|
Withdrawal of medical care |
139 (7.1) |
99 (7.6) |
40 (6.1) |
0.24 |
404 (12.4) |
21 (6.7) |
|
Surgical treatment |
366 (18.6) |
251 (19.2) |
115 (17.6) |
0.39 |
206 (6.3) |
43 (13.7) |
|
Length of hospital stay |
16 (8-22) |
16 (9-22) |
16 (8-22) |
0.99 |
18 (11-26) |
14 (12-18) |
|
In-hospital neurological deterioration |
373 (19.0) |
250 (19.1) |
123 (18.8) |
0.87 |
476 (14.6) |
18 (5.7) |
Table 2.
Scoring system of the intracerebral hemorrhage progression score
|
Item |
Score |
|
Age ≥80 years |
2 |
|
Male sex (yes) |
2 |
|
History of diabetes mellitus (yes) |
2 |
|
History of atrial fibrillation (yes) |
7 |
|
Admission GCS score ≤8 (yes) |
6 |
|
Dysphagia on admission (yes) |
3 |
|
Infratentorial hematoma location (yes) |
2 |
|
Hematoma volume (mL) |
|
|
Superatentorial ≤39 or infratentorial ≤4 |
0 |
|
Superatentorial 40-69 or infratentorial 5-10 |
4 |
|
Superatentorial ≥70 or infratentorial ≥11 |
5 |
|
Blood glucose >11.1 mmol/L |
3 |
|
Total |
32 |
References
1. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association.
Circulation 2021;143:e254-e743.

2. Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association.
Stroke 2015;46:2032-2060.


3. Steiner T, Al-Shahi Salman R, Beer R, Christensen H, Cordonnier C, Csiba L, et al. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage.
Int J Stroke 2014;9:840-855.



4. Wang HL, Hsu WY, Lee MH, Weng HH, Chang SW, Yang JT, et al. Automatic machine-learning-based outcome prediction in patients with primary intracerebral hemorrhage.
Front Neurol 2019;10:910.



5. Okazaki S, Yamamoto H, Foster LD, Fukuda-Doi M, Koga M, Ihara M, et al. Late neurological deterioration after acute intracerebral hemorrhage: a post hoc analysis of the ATACH-2 trial.
Cerebrovasc Dis 2020;49:26-31.



7. You S, Zheng D, Delcourt C, Sato S, Cao Y, Zhang S, et al. Determinants of early versus delayed neurological deterioration in intracerebral hemorrhage.
Stroke 2019;50:1409-1414.


8. Ji R, Wang W, Liu X, Wang L, Jiang R, Zhang R, et al. Headto-head comparison of prognostic models of spontaneous intracerebral hemorrhage: tools for personalized care and clinical trial in ICH.
Neurol Res 2022;44:146-155.


9. Wang Y, Cui L, Ji X, Dong Q, Zeng J, Wang Y, et al. The China National Stroke Registry for patients with acute cerebrovascular events: design, rationale, and baseline patient characteristics.
Int J Stroke 2011;6:355-361.



10. Ji R, Li G, Zhang R, Hou H, Zhao X, Wang Y. Higher risk of deep vein thrombosis after hemorrhagic stroke than after acute ischemic stroke.
J Vasc Nurs 2019;37:18-27.













