 |
 |
- Search
| J Stroke > Volume 24(3); 2022 > Article |
|
Abstract
Background and Purpose
Many patients with stroke cannot receive intravenous thrombolysis because the time of symptom onset is unknown. We tested whether a simple method of computed tomography (CT)-based quantification of water uptake in the ischemic tissue can identify patients with stroke onset within 4.5 hours.
Methods
This retrospective analysis of the MissPerfeCT study (August 2009 to November 2017) includes consecutive patients with known onset of symptoms from seven tertiary stroke centers. We developed a simplified algorithm based on region of interest (ROI) measurements to quantify water uptake of the ischemic lesion and thereby quantify time of symptom onset within and beyond 4.5 hours. Perfusion CT was used to identify ischemic brain tissue, and its density was measured in non-contrast CT and related to the density of the corresponding area of the contralateral hemisphere to quantify lesion water uptake.
Results
Of 263 patients, 204 (77.6%) had CT within 4.5 hours. Water uptake was significantly lower in patients with stroke onset within (6.7%; 95% confidence interval [CI], 6.0% to 7.4%) compared to beyond 4.5 hours (12.7%; 95% CI, 10.7% to 14.7%). The area under the curve for distinguishing these patient groups according to percentage water uptake was 0.744 with an optimal cut-off value of 9.5%. According to this cut-off the positive predictive value was 88.8%, sensitivity was 73.5%, specificity 67.8%, negative predictive value was 42.6%.
Current guidelines recommend thrombolysis with intravenous alteplase (IV-tPA) for acute ischemic stroke up to 4.5 hours from stroke onset [1]. Following these guidelines patients with unknown time of symptom onset, representing up to 25% of all stroke patients, are excluded from IV-tPA treatment [2]. There are currently two imaging approaches to identify those patients likely to benefit from IV-tPA treatment among those “wake-up stroke” patients. The first approach uses the identification of the potentially salvageable penumbra by perfusion imaging [3]. The second approach quantifies the mismatch between diffusion weighted imaging (DWI) and fluid attenuated inversion recovery (FLAIR) sequences on magnetic resonance imaging (MRI) to identify those patients with a symptom onset of less than 4.5 hours [4]. Both imaging methods have crucial limitations; the first approach requires the application of dedicated software and is reported to identify a lower proportion of patients compared to the “tissue-clock” approach [5]. The second approach is limited by the restricted availability and increased logistic effort of MRI for acute stroke triage.
Recently, we have established a computed tomography (CT) based method for the quantification of lesion water uptake on admission that is able to identify patients within the 4.5-hour-time window with high accuracy [6]. The aim of this study was to develop a simple method that identifies patients within the 4.5-hour-time window based on simple region of interest (ROI) measurements as a proxy of lesion water uptake in admission CT. This approach is easily and rapidly applicable with standard radiological software worldwide and without requiring additional software tools. We therefore performed a multicenter cohort study including patients with known symptom onset and hypothesized that our ROI-based method would be able to identify those patients in the 4.5-hour-time window eligible for thrombolysis.
This analysis of the retrospective multicenter MissPerfeCT study (August 2009 to November 2017) includes consecutive patients with a known onset of symptoms from seven tertiary stroke centers who were clinically diagnosed with acute ischemic stroke and received multimodal CT on admission including standard non-contrast computed tomography (NCCT), computed tomography angiography (CTA), and computed tomography perfusion (CTP). Consecutive patients were included to reduce the risk of bias. Consecutive patients from the following university medical centers were included: Bochum (October 2016 to August 2017), Goettingen (October 2016 to November 2017), Dresden (May 2015 to December 2016), Greifswald (September 2015 to October 2017), Luebeck (March 2015 to December 2016), LMU Munich (August 2009 to June 2012), Muenster (May 2016 to November 2017). Inclusion criteria were: (1) evidence of acute intracranial vessel occlusion (any supratentorial proximal or peripheral artery of the anterior cerebral artery, middle cerebral artery, or posterior cerebral artery territory) by ischemic perfusion deficit and/or CT hyperdense thrombus and/or CTA vessel occlusion; (2) acute symptoms attributable to the ischemic CTP lesion; and (3) sufficient NCCT quality for judgment of early ischemic hypodensity (potential limitations were old infarcts, severe white matter disease and movement artifacts), sufficient CTP quality for judgment of the ischemic core lesion (potential limitations were insufficient contrast bolus or movement artifacts).
The study was approved by the local ethics committees of all centers. All study protocols and procedures were conducted in accordance with the Declaration of Helsinki. The MissPerfeCT study is registered with clinicaltrials.gov (NCT04277728). This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Patients received NCCT, CTA, and whole brain CTP performed on 64 or 128 dual slice scanners (Siemens Definition AS+; Siemens Definition Flash, Siemens Healthcare, Forchheim, Germany; Philips Brilliance 64, Philips Medical Systems, Eindhoven, Netherlands)—CT: 120 kV, 280 to 320 mA, 5.0 mm slice reconstruction; CTA: 100 to 120 kV, 260 to 300 mA, 1.0 mm slice reconstruction, 5 mm MIP reconstruction with 1 mm increment; CTP: 80 kV, 200 to 250 mA, 5 mm slice reconstruction (maximum 10 mm), slice sampling rate 1.50 seconds (minimum 1.33 seconds), scan time 45 seconds (maximum 60 seconds), biphasic injection with 30 mL (maximum 40 mL) of highly iodinated contrast medium with 350 mg iodine/mL (maximum 400 mg/mL) injected with at least 4 mL/sec (maximum 6 mL/sec) followed by a 30 mL NaCl chaser bolus. All perfusion parameter maps were calculated on a dedicated workstation (Syngo VE52A with VPCT-Neuro, Siemens Healthcare, Forchheim, Germany) based on a deconvolution model by least mean squares fitting including cerebral blood volume (CBV), cerebral blood flow, mean transit time, and time to drain [7].
The imaging biomarker for lesion age, the water uptake (w), is based on ischemic and preischemic CT density and was calculated according to the following formula:
as previously published [6]. The algorithm to classify patients within the 4.5-hour-time window was based on placement of a ROI in the area of the NCCT that corresponded with the region with the lowest CBV. The ROI was chosen as large as possible but at least 1 cm2. As a reference a ROI of the same size was placed in the contralateral corresponding region in the NCCT and the ratio of the affected/unaffected side was calculated. Placement of the ROIs was done independently by two experienced readers (P.B.S. and M.H.) blinded to clinical information (Figure 1 illustrates the method). When judgements were conflicting, images were rated by a third reader (J.M.) and then discussed until consensus was reached between all observers on the optimal ROI placement. The consensus judgement was used for further analysis.
Patients within and beyond 4.5 hours were compared by use of Pearson chi-square test for categorial variables and Student t-test or Mann-Whitney U test for continuous variables. We calculated the area under the curve (AUC), sensitivity, specificity, positive predictive value and negative predictive value (with Wilson 95% confidence interval [CI]) for the identification of patients within 4.5 hours of symptom onset. Interobserver agreement based on Cohen’s kappa was calculated for both readers after ROI placement. Statistical analyses were carried out using SPSS version 26 (IBM Co., Armonk, NY, USA).
A total of 263 patients were included in our study of whom 204 (77.6%) presented within and 59 (22.4%) patients later than 4.5 hours of symptom onset. Table 1 shows their baseline characteristics. Groups for patients presenting <4.5 and >4.5 hours were comparable regarding age (mean±standard deviation, 72.3±13.3 years vs. 68.6±13.2 years, P=0.065), gender (women, 52.0% vs. 50.8, P=0.526), and National Institutes of Health Stroke Scale score on admission (median 12.0 [interquartile range, IQR, 9.8 to 14.2] vs. 12.0 [IQR, 9.5 to 14.4], P=0.416). Comorbidities like hypertension (80.3% vs. 73.2%, P=0.252), diabetes mellitus (23.7% vs. 26.8%, P=0.639), and atrial fibrillation (51.0% vs. 39.3%, P=0.121) were also not significantly different between the groups.
Median ratio of Hounsfield units (HU) for patients ≤4.5 hours was 6.7% (95% CI, 6.0% to 7.4%) and 12.7% (95% CI, 10.7% to 14.7%) for patients with an onset of >4.5 hours. The AUC discriminating patients within from those beyond 4.5 hours of symptom onset according to percentage of water uptake was 0.744 (Figure 2). Interobserver agreement for ROI placement was 90.9 % with a κ of 0.807 (95% CI, 0.701 to 0.913) indicating an ‘almost perfect’ agreement. The ideal cut-off value for achieving the highest positive predictive value of 88.8% in our study was 0.095 (9.5% water uptake) leading to a sensitivity of 73.5%, a specificity of 67.8%, and a negative predictive value of 42.6%, respectively (Figure 3).
This multicenter study shows that a simple ROI-based assessment of admission CTs identifies patients within 4.5 hours of symptom onset, thus eligible for thrombolysis. As discussed in the DWI-FLAIR mismatch for the identification of patients with acute ischemic stroke within 4.5 hours of symptom onset (PREFLAIR) study, the observational study preceding the randomized Efficacy and Safety of MRI-Based Thrombolysis in Wake-Up Stroke (WAKE-UP) trial, the positive predictive value is likely to be most relevant measure for clinical practice [8]. Thus, the ideal cut-off with a maximum positive predictive value of 88.6% would be 0.095 meaning a water uptake of 9.5% in our study. We have recently shown that the CT-based quantification of brain tissue water uptake identifies stroke patients with symptom onset within 4.5 hours with high accuracy [6]. The pathophysiological basis for this observation is that tissue water uptake after cerebral artery occlusion follows a characteristic course that is visualized by a decreasing CT density [9-11]. However, in our previous study we used specific post-processing software to quantify water uptake within early infarct lesion that is not widely available in routine practice. We therefore developed the concept of a ROI-based assessment that translates the pathophysiological approach of water uptake quantification into a clinically feasible and fast applicable algorithm not requiring a specific software.
Until recently, imaging strategies for determining the time of symptom onset were primarily based on MRI, specifically, the DWI-FLAIR mismatch concept [8]. Following this concept patients with visible changes on DWI but normal FLAIR are likely within the time window of thrombolysis. The multicenter, randomized, double-blind WAKE-UP trial showed that thrombolysis in patients with unknown time of symptom onset guided by MRI DWI-FLAIR mismatch results in a significantly better functional outcome [4]. Overall, the WAKE-UP trial confirms the rationale of the “tissue-clock” approach for the identification of stroke patients with unknown time of symptom onset eligible for thrombolysis. However, compared with MRI, CT is less affected by contraindications (like pacemakers) and is more generally available in the acute setting in most hospitals that treat acute ischemic stroke patients and thus remains the primary imaging modality worldwide.
An alternative approach to identify patients who may benefit from thrombolysis despite unknown time of symptom onset is based on imaging a favorable penumbral pattern in MRI or CT with infarct core-perfusion mismatch as a surrogate of salvageable tissue [3]. A recent systematic review and meta-analysis showed that patients with a favorable penumbral pattern operationally defined by automated software in core laboratory analysis had a significant benefit from thrombolysis [12]. However, automated imaging software required for perfusion mismatch quantification used in clinical studies limits its use in clinical practice. In addition, a recent MRI study found that the yield of a “tissue-clock” imaging approach to select patients eligible for thrombolysis in the unknown time window was double that of infarct core-perfusion mismatch based patient selection [5]. In this context, our ROI-based method has several advantages over existing imaging based methods [13] for the identification of patients with unknown time of symptom onset eligible for thrombolysis including the speedy accessibility of CT worldwide compared to MRI and the dispensability of automated software tools.
A limitation of our study is the retrospective design. However, all images were assessed by readers masked to clinical information. Moreover, the cut-off in this study was not validated in an external cohort. Further, the use of different perfusion imaging processing softwares may lead to distinct findings regarding ischemic volumes on CTP maps [14]. A strength of our study is the almost perfect interobserver agreement for ROI placement that was much better than the interobserver agreement reported for DWI-FLAIR mismatch detection on MRI [8].
In conclusion, ischemic stroke patients with an unknown time of symptom onset can be identified as being within 4.5 hours after symptom onset using a simple ROI-based method of assessment of admission CT. This can potentially guide the decision to administration of thrombolysis in patients with unknown time of stroke onset but the findings need to be validated by prospective, large-scale studies.
Notes
Disclosure
Jens Minnerup has received grants from Deutsche Forschungsgemeinschaft, Bundesministerium für Bildung und Forschung (BMBF), Else Kröner-Fresenius-Stiftung, EVER Pharma Jena GmbH, and Ferrer International, travel grants from Boehringer Ingelheim, and speaking fees from Bayer Vital and Chugai Pharma; Jens Fiehler has received grants from German Ministry of Science and Education (BMBF), German Ministry of Economy and Innovation (BMWi), German Research Foundation (DFG), European Union (EU), Hamburgische Investitions- und Förderbank (IFB), Medtronic, Microvention, Route92, Stryker. Consultant for: Acandis, Bayer, Boehringer Ingelheim, Cerenovus, Evasc Neurovascular, MD Clinicals, Medtronic, Microvention, Penumbra, Phenox, Stryker, Transverse Medical. Stock holder: Tegus Medical; Marco Duering has received honoraria for lectures from Bayer Vital and Sanofi Genyzme. Consultant for Hovid Berhad and Roche Pharma; Christos Krogias has received honoraria and travel grants from Bayer Vital and Daiichi-Sankyo; Daniel Kaiser receives a grant from Else Kröner-Fresenius-Center for Digital Health; Peter Schramm has received grants from Siemens and Penumbra. Consultant for: Penumbra, Phenox, Stryker, Cerus endovascular.
The other authors have no financial conflicts of interest.
Figure 1.
Illustration of the method of quantification of lesion water uptake for a patients with a symptom onset <4.5 and >4.5 hours. (A) Cerebral blood volume (CBV)-map (mL/100 g) showing low CBV lesion in the right posterior cerebral artery territory. (B) A region of interest (yellow circles) is placed in the area that corresponds to the lowest CBV lesion in the non-contrast computed tomography (NCCT). Mean computed tomography (CT) attenuation in of the lesion on the ischemic side (41.7 Hounsfield unit [HU]) is divided through mean attenuation on the contralateral side (42.1 HU) indicating a quotient of 0.99 which correctly determined the patient to be inside the 4.5-hour-time window. (C) CBV-map (mL/100 g) showing low CBV lesion in the right temporal middle cerebral artery territory. (D) A region of interest (yellow circles) is placed in the area that corresponds to the lowest CBV lesion in the NCCT. Mean CT attenuation of the lesion on the ischemic side (25.9 HU) is divided through mean attenuation on the contralateral side (36.8 HU) indicating a quotient of 0.70 which correctly determined the patient to be outside the 4.5-hour-time window.
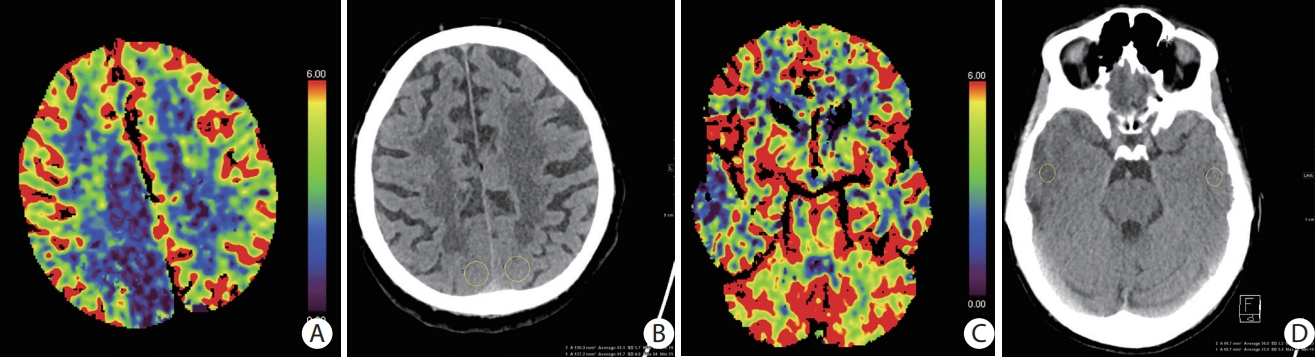
Figure 2.
Receiver operating characteristic (ROC)-curve for identification of patients within 4.5 hours of symptom onset. AUC, area under the curve.

Figure 3.
Cut-off 0.095 for identification of patients within 4.5 hours of symptom onset. PPV, positive predictive value; NPV, negative predictive value.
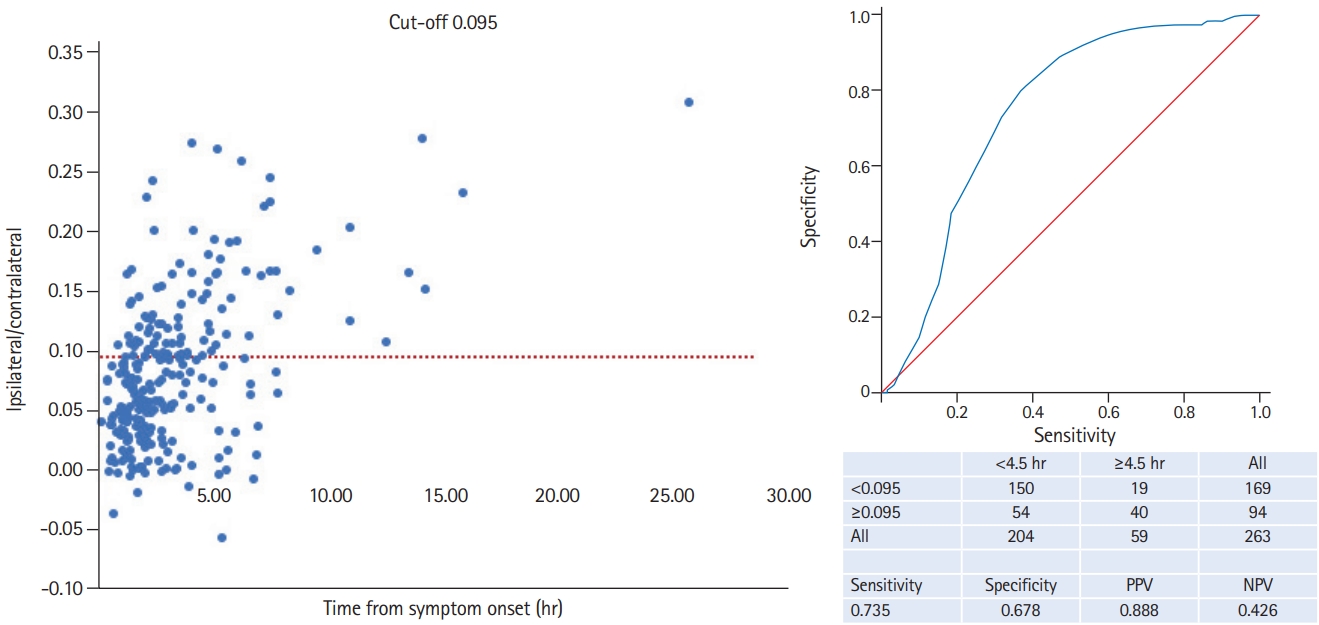
Table 1.
Baseline characteristics of patients
| Characteristic | Patients with CT within 4.5 hours (n=204) | Patients with CT after 4.5 hours (n=59) | P |
|---|---|---|---|
| Demographics | |||
| Age (yr) | 72.3±13.3 | 68.6±13.2 | 0.065 |
| Women | 106 (52.0) | 30 (50.8) | 0.526 |
| Comorbidities | |||
| Hypertension | 159 (80.3) | 41 (73.2) | 0.252 |
| Missing | 6 | 3 | |
| Diabetes mellitus | 47 (23.7) | 15 (26.8) | 0.639 |
| Missing | 6 | 3 | |
| CAD | 34 (23.0) | 14 (31.8) | 0.234 |
| Missing | 56 | 15 | |
| Atrial fibrillation | 101 (51.0) | 22 (39.3) | 0.121 |
| Missing | 6 | 3 | |
| Hypercholesterolemia | 48 (34.8) | 23 (51.1) | 0.051 |
| Missing | 66 | 14 | |
| Smoker | 39 (19.7) | 15 (27.3) | 0.225 |
| Missing | 6 | 4 | |
| Stroke etiology | 0.139 | ||
| Atherothrombotic | 38 (19.2) | 13 (23.2) | |
| Small vessel | 7 (3.5) | 1 (1.8) | |
| Cardioembolic | 111 (56.1) | 23 (41.1) | |
| Undetermined aetiology | 39 (19.7) | 16 (28.6) | |
| Other aetiology | 3 (1.5) | 3 (5.4) | |
| Missing | 6 | 3 | |
| NIHSS score | |||
| Median (IQR) | 12.0 (9.8-14.2) | 12.0 (9.5-14.4) | 0.416 |
| Mean±SD | 12.3±6.9 | 13.2±6.91 | 0.387 |
| Missing* | 8 | 3 | |
| Time from symptom onset to CT (min) | 123±59 | 430±217 | <0.001 |
References
1. Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014;384:1929-1935.



2. Fink JN, Kumar S, Horkan C, Linfante I, Selim MH, Caplan LR, et al. The stroke patient who woke up: clinical and radiological features, including diffusion and perfusion MRI. Stroke 2002;33:988-993.


3. Ma H, Campbell BC, Parsons MW, Churilov L, Levi CR, Hsu C, et al. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med 2019;380:1795-1803.


4. Thomalla G, Simonsen CZ, Boutitie F, Andersen G, Berthezene Y, Cheng B, et al. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med 2018;379:611-622.


5. Scheldeman L, Wouters A, Boutitie F, Dupont P, Christensen S, Cheng B, et al. Different mismatch concepts for magnetic resonance imaging-guided thrombolysis in unknown onset stroke. Ann Neurol 2020;87:931-938.



6. Minnerup J, Broocks G, Kalkoffen J, Langner S, Knauth M, Psychogios MN, et al. Computed tomography-based quantification of lesion water uptake identifies patients within 4.5 hours of stroke onset: a multicenter observational study. Ann Neurol 2016;80:924-934.



7. Kemmling A, Flottmann F, Forkert ND, Minnerup J, Heindel W, Thomalla G, et al. Multivariate dynamic prediction of ischemic infarction and tissue salvage as a function of time and degree of recanalization. J Cereb Blood Flow Metab 2015;35:1397-1405.




8. Thomalla G, Cheng B, Ebinger M, Hao Q, Tourdias T, Wu O, et al. DWI-FLAIR mismatch for the identification of patients with acute ischaemic stroke within 4·5 h of symptom onset (PRE-FLAIR): a multicentre observational study. Lancet Neurol 2011;10:978-986.


9. Breuer L, Schellinger PD, Huttner HB, Halwachs R, Engelhorn T, Doerfler A, et al. Feasibility and safety of magnetic resonance imaging-based thrombolysis in patients with stroke on awakening: initial single-centre experience. Int J Stroke 2010;5:68-73.



10. Aoki J, Kimura K, Iguchi Y, Shibazaki K, Iwanaga T, Watanabe M, et al. Intravenous thrombolysis based on diffusion-weighted imaging and fluid-attenuated inversion recovery mismatch in acute stroke patients with unknown onset time. Cerebrovasc Dis 2011;31:435-441.



11. Dzialowski I, Weber J, Doerfler A, Forsting M, von Kummer R. Brain tissue water uptake after middle cerebral artery occlusion assessed with CT. J Neuroimaging 2004;14:42-48.


12. Campbell BC, Ma H, Ringleb PA, Parsons MW, Churilov L, Bendszus M, et al. Extending thrombolysis to 4·5-9 h and wakeup stroke using perfusion imaging: a systematic review and meta-analysis of individual patient data. Lancet 2019;394:139-147.









