Stroke in Women: A Review Focused on Epidemiology, Risk Factors, and Outcomes
Article information
Abstract
Stroke is a particularly important issue for women. Women account for over half of all persons who experienced a stroke. The lifetime risk of stroke is higher in women than in men. In addition, women have worse stroke outcomes than men. Several risk factors have a higher association with stroke in women than in men, and women-specific risk factors that men do not have should be considered. This focused review highlights recent findings in stroke epidemiology, risk factors, and outcomes in women.
Introduction
Globally, stroke remained the second-leading cause of death and the third-leading cause of disability and death combined in 2019 [1]. Stroke is a particularly important issue for women, who account for over half (56% in 2019) of all persons who have experienced a stroke [2]. Women have higher lifetime risk (LTR) of stroke [3] and poorer stroke outcome [4] than men. In women, the distribution of some risk factors and the risk of stroke with those risk factors are different from men. Women also have unique risk factors that men do not have. In this review, we present a focused and updated review of stroke epidemiology, risk factors, and outcomes in women.
Epidemiology
Incidence of stroke
A lower age-adjusted incidence of stroke in women than in men has been consistently reported [5-7]. It has been generally accepted that the stroke incidence is much lower in women than in men until well after menopause in older women [5]. However, inconsistent with this previous knowledge, recent studies have reported that women have a higher or similar stroke incidence as compared to men in younger age strata (Table 1) [6-9]. After that, in middle age, men show a higher stroke incidence than women [5-8]. The difference in stroke incidence between men and women narrows as the stroke incidence increases in postmenopausal women, and eventually, the stroke incidence in women is close to [5-8] or even higher [10] than men in the eighth decade. Particularly in older women, significant disparities by race/ethnicity were reported in NOMAS (Northern Manhattan Study) [11]. For example, Black and Hispanic women aged ≥70 years had a 76%–77% higher risk of stroke compared with White women after adjustment for age, education, and insurance status, while no differences were observed in men [11].
Globally, the age-standardized incidence rate of stroke decreased between 1990 and 2019 by 17% [1]; although there are regional variations across countries and greater burdens in low- and middle-income countries than in high-income countries [1,12]. In the GCNKSS (Greater Cincinnati/Northern Kentucky Stroke Study) with a large population representative of the United States (US), the overall stroke incidence between 1993/1994 and 2015 declined significantly for both sexes: the declines were from 229 to 174 (per 100,000) for women and from 282 to 211 (per 100,000) for men [6]. The ARIC (Atherosclerosis Risk in Communities) study reported that the stroke incidence rates in adults ≥65 years decreased by 32% per 10 years during the 30-year period from 1987 to 2017, and the decreases were similar between men and women [13].
Lifetime risk of stroke
It is generally considered that the LTR of stroke is higher in women than in men, mainly due to their longer life expectancy. According to the estimation from the Framingham Study, the LTR in middle-aged adults is approximately 1 in 5 (20%–21%) for women and 1 in 6 (14%–17%) for men [3]. In the Global Burden of Disease (GBD) study, the global LTR of stroke from the age of 25 years onward was 25.1% for women and 24.7% for men in 2016 [14], but there were geographic variations with the highest LTRs among women in Eastern Europe (36.5%) and East Asia (36.3%) [14].
Stroke subtype
Contrary to other stroke subtypes, subarachnoid hemorrhage (SAH) tends to be more common in women than in men [7]. In a recent study from Canada, women had a higher hazard of SAH compared to men (hazard ratio [HR] [95% confidence interval (CI)], 1.29 [1.24–1.33]), whereas had a lower hazard of ischemic stroke (0.78 [0.77–0.79]), transient ischemic attack (TIA) (0.85 [0.84–0.86]), and intracerebral hemorrhage (ICH) (0.76 [0.74–0.78]) [7]. Intracranial aneurysm is more prevalent in women than men [15,16]. Female sex has also been reported as a risk factor for de novo aneurysm formation and growth of aneurysms [16]. In a recent meta-analysis, female sex was associated with greater odds of aneurysmal SAH (HR [95% CI], 1.90 [1.47–2.46]) [17]. Decreased estrogen levels after menopause have been suspected to affect the increased risk of aneurysm formation and rupture in women [16].
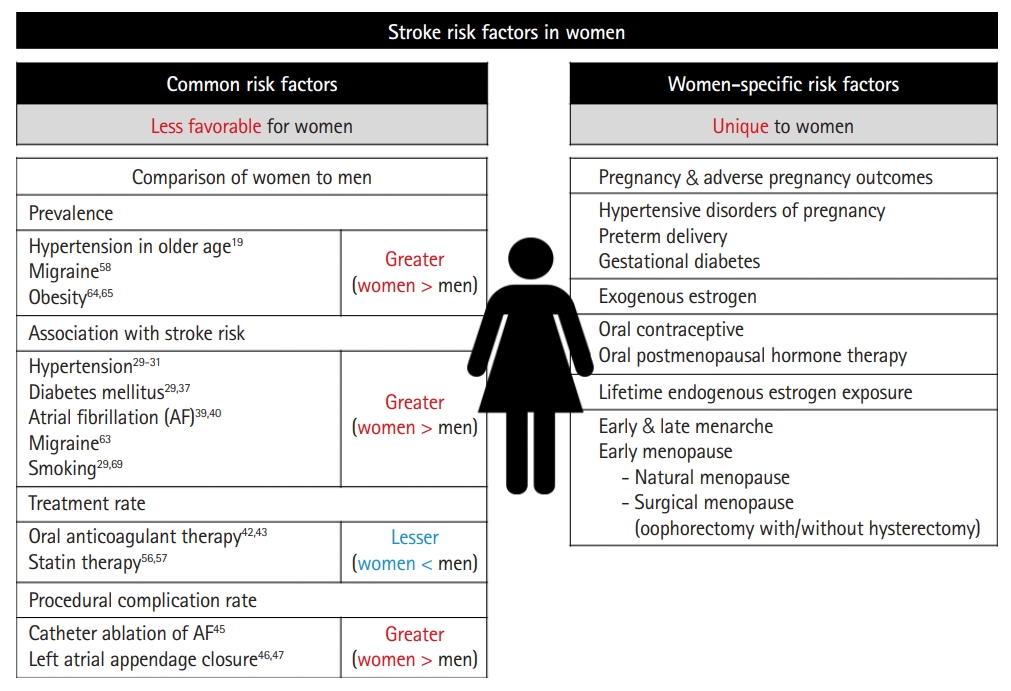
Risk factors (Figure 1)
Common risk factors
Hypertension
Hypertension (HTN) is the most prevalent risk factor for stroke. In 2019, the global age-standardized prevalence of HTN (defined as having systolic/diastolic blood pressure [SBP/DBP] ≥140/90 mm Hg or taking medication for HTN) was 34% for men and 32% for women [18]. Although the overall prevalence of HTN is higher in men than in women [18,19], the prevalence according to age shows a different trend [19]. As age increases, the prevalence of HTN increases in both men and women; however, the prevalence of HTN in postmenopausal women rises steeply, with the prevalence in women eventually exceeding that in men [19]. Similarly, the association between age and SBP in men and women who were not receiving antihypertensive treatment showed a linear increase, with the mean blood pressure (BP) in women exceeding that in men at 75 years of age in the May Measurement Month (MMM) 2018 report [20].
Regarding the awareness, treatment, and control rates of HTN, there have been many reports that these are higher in women than in men [18,19,21-25]. Globally, the treatment rate was 47% in women and 38% in men, and BP was controlled in less than half of those who received treatment, leading to global control rates of 23% for women and 18% for men with HTN [18]. However, since the awareness, treatment, and control rates are affected by various factors other than sex such as age and regional factors including health care system, race/ethnicity, and socioeconomic and cultural differences, these factors should be considered together [18-26]. For example, the overall sex difference in the control rate of HTN (women > men) is mainly due to the difference at younger ages (defined variously by studies from <40 to <60 years of age), and this sex gap diminishes with increasing age [22-25]. In reports from US and South Korea, the control rates were even reversed (men > women) from the age group ≥60 years [22,25].
HTN is the risk factor that has the greatest influence on the risk of stroke in both men and women. The INTERSTROKE (Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries) study suggested that 10 common risk factors could explain approximately 90% of the population attributable risk (PAR) for stroke [27]. Among the 10 risk factors, HTN was the factor contributing the most, and the PAR in women and men was 52.3% and 45.2%, respectively [27]. Regarding sex differences in the effect of HTN on stroke risk, previous and recent studies have shown different results. A previous meta-analysis demonstrated a similar impact of BP on the risk of stroke in both sexes; for every 10 mm Hg increase in SBP, stroke risk increased by ≈25% [28]. However, in a recent large population-based cohort study from the UK, HTN was associated with a greater HR of stroke in women than men with a women-to-men ratio of HR of 1.36 (95% CI, 1.26–1.47) [29]. The REGARDS (Reasons for Geographic and Racial Differences in Stroke) study from the US found that the association between the increase in HTN severity and incident ischemic stroke was almost twice as great in women as in men [30]. A recent large community-based cohort study (n=27,542, 54% women) suggested that the risk of stroke begins at lower thresholds of SBP for women than for men [31]. In this study, the stroke risk for women with an SBP of 120–129 mm Hg was comparable to the risk in men with an SBP of 140–149 mm Hg.
Diabetes mellitus
The global prevalence of diabetes mellitus (DM) in 2019 was estimated to be 9.3% (adults aged 20–79 years) and was reported to be slightly higher in men (9.6%) than in women (9.0%) [32]. Regarding the treatment and control rates of DM, the results have not been consistent between studies. According to the data from the US National Health and Nutrition Examination Survey, the treatment and control rates were similar or higher in women than men (81% and 30%, respectively, for women and 80% and 20%, respectively, for men in 2013–2016) [33]. In contrast to the trend in this study, in another study from Italy, the rate of achievement of target hemoglobin A1C (<7%) was lower in women (33.8%) than in men (40.2%) [34].
DM is an important risk factor for stroke with HRs (95% CI) of 2.27 (1.95–2.65) for ischemic stroke and 1.56 (1.19–2.05) for hemorrhagic stroke according to a meta-analysis from 2010 [35]. Among the ischemic stroke subtypes, DM is particularly closely related to the risk of lacunar stroke [36]. The effect of DM on stroke risk is greater than in women than in men. In a pooled analysis of 64 cohorts, women with DM had a 27% greater relative risk (RR) of stroke than men with DM [37]. In the UK Biobank cohort study, DM was associated with a higher HR of ischemic stroke in women than men with a women-to-men ratio of HR of 1.25 (95% CI, 1.00–1.56) [29]. DM was also associated with a higher RR of death after ischemic stroke in women compared to men (RR [95% CI], 3.16 [2.44–4.10] vs. 2.20 [1.75–2.77]) in a previous meta-analysis [38].
Atrial fibrillation
Women have lower age-adjusted incidence and prevalence of atrial fibrillation (AF) than men [39]. However, because of the longer life expectancy for women, the LTR of AF for women and the absolute number of women with AF are similar to those of men [39]. Female sex is considered as a risk factor for AF-related systemic thromboembolism including stroke; for this reason, female sex itself is included as a variable in AF risk stratification models such as the CHA2DS2-VASc score [39]. In a meta-analysis of 30 cohort studies, AF was associated with a nearly two-fold higher risk of stroke in women than in men with a women-to-men ratio of RR of 1.99 (95% CI, 1.46–2.71) [40]. In the UK Biobank cohort study, AF was also associated with a higher HR of hemorrhagic stroke in women than in men with a women-to-men ratio of HRs of 2.80 (95% CI, 1.07–7.36) [29]. There is also the view that female sex is a risk modifier rather than a risk factor for stroke in AF [41].
Despite the higher AF-related thromboembolism risk, women with AF show lower oral anticoagulation (OAC) use than men. In an analysis of the PINNACLE (Practice Innovation and Clinical Excellence) registry (n=691,906, 48.5% women), women were significantly less likely to receive OAC overall (56.7% vs. 61.3%; P<0.001) and at all levels of CHA2DS2-VASc score [42]. More recent study has also shown consistent results, and lower OAC use may partially mediate the increased risk of stroke in women [43]. Women are also less likely to undergo catheter ablation of AF [44]. Furthermore, women who underwent catheter ablation of AF experience lower efficacy and a higher risk of stroke/TIA and major complications than men [45]. Recent studies have also shown that women are more likely to experience worse periprocedural outcomes after left atrial appendage closure [46,47].
Dyslipidemia
The prevalence of dyslipidemia has been generally reported to be higher in men or similar in men and women [33,48]. According to the data from the US National Health and Nutrition Examination Survey, a higher percentage of men than women with cardiovascular disease (CVD) had dyslipidemia, whereas rates were similar for those without CVD [33]. Dyslipidemia is a risk factor for ischemic stroke, especially for stroke due to large artery atherosclerosis [49-51]. In most studies, there is an association between higher total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) levels and increased ischemic stroke risk [50,51]. In a prospective women’s cohort study of more than 27,000 US women aged ≥45 years, when comparing the highest to the lowest quintile, the multivariable-adjusted HRs of ischemic stroke were 2.27 (95% CI, 1.43–3.60) for TC and 1.74 (95% CI, 1.14–2.66) for LDL-C [52]. Regarding sex differences in the association between dyslipidemia and stroke, there were no appreciable sex differences regarding the association between TC and ischemic stroke in a previous meta-analysis of data from 61 cohorts (n=577,642, 11,914 stroke cases) [53]. In the UK Biobank cohort study, there was also no evidence of a sex difference in the associations between lipid profiles (TC, LDL-C, and high-density lipoprotein cholesterol) and stroke [29].
Statin therapy has many benefits for ischemic stroke [54]. Statin therapy shows similar effectiveness for the prevention of major vascular events in men and women at an equivalent risk of CVD [55]. Despite the similar effectiveness of statin therapy in men and women, women are less likely than men to be treated with statins in real practice [56,57]. In the PALM (The Patient and Provider Assessment of Lipid Management) registry, a US nationwide registry of outpatients with or at risk for atherosclerotic CVD, women were less likely than men to be prescribed any statin therapy (67.0% vs. 78.4%; P<0.001) or to receive a statin at the guideline-recommended intensity (36.7% vs. 45.2%; P<0.001) [57].
Migraine
Migraine is more prevalent in women than in men. According to the GBD study, for 2016, the global age-standardized prevalence of migraine was 14.4% overall: 18.9% for women vs. 9.8% for men [58]. Migraine, particularly migraine with aura, is associated with an increased risk of stroke. The possible mechanisms explaining the migraine-stroke connection include genetic predisposition, endothelial dysfunction, coagulation abnormalities, arterial dissection, and paradoxical embolism via a patent foramen ovale (PFO) [59]. In a meta-analysis of 16 cohort studies, migraine with aura was associated with a higher risk of stroke with an adjusted HR of 1.56 (95% CI, 1.30–1.87); however, this finding was not evident in migraine without aura [60]. The association of ischemic stroke risk with migraine was consistently observed, but conflicting results were shown for hemorrhagic stroke: some studies have found an increased risk for hemorrhagic stroke in migraineurs [60,61], while others have found no association [62,63]. Women showed a higher association between migraine and ischemic stroke than men (RR [95% CI], 2.08 [1.13–3.84] vs. 1.37 [0.89–2.11]), and this was especially higher in young women under the age of 45 (RR [95% CI], 3.65 [2.21–6.04]) and women using oral contraceptives (RR [95% CI], 7.02 [1.51–32.68]) [63]. The association between migraine and ischemic stroke was stronger in smokers than in non-smokers (RR [95% CI], 9.03 [4.22–19.34] vs. 1.56 [0.41–5.85]) [63].
Obesity
The prevalence of obesity is greater for women than for men [64,65]. Obesity is a risk factor for ischemic stroke in both men and women [29,66]. In the UK Biobank cohort study, obesity was associated with an increased risk of total stroke of ≈30% in both sexes with a stronger association in women than men for ischemic stroke (women-to-men ratio of HR [95% CI], 1.36 [1.21–1.54]) [29]. However, for hemorrhagic stroke, only men showed a significant association in this study [29]. A UK prospective study in women found that a higher body mass index was associated with an increased risk of ischemic stroke but a decreased risk of hemorrhagic stroke in women [67].
Smoking
The prevalence of smoking is lower in women than in men [33,68]. According to the meta-analysis including 14 studies involving 303,134 subjects, smokers had an overall increased risk of stroke compared with non-smokers with an odds ratio (OR) of 1.61 (95% CI, 1.34–1.93), and there was a dose-dependent relationship between current smoking status and the risk of stroke: the risk was increased by 12% for each increment of 5 cigarettes per day [69]. A meta-analysis published in 2013 showed that smoking-related stroke risk was similar for men and women, and only Western women (not Asian women) showed a greater risk than men [70]. However, a more recent meta-analysis showed a greater smoking-related stroke risk in women than in men (OR [95% CI], 1.88 [1.45–2.44] vs. 1.54 [1.11–2.13]) [69]. In the UK Biobank cohort study, current smokers have a considerably higher risk of stroke than never smokers, with evidence to suggest that the excess risk of stroke is greater for women than men (women-to-men ratio of HR [95% CI], 1.18 [1.02–1.36]) [29].
Women-specific risk factors
Pregnancy
Maternal stroke
Pregnancy-related stroke, commonly called maternal stroke, is a term for ischemic or hemorrhagic strokes that occur during pregnancy and the postpartum period (defined variously by studies as up to 12 weeks after delivery). In a meta-analysis including 11 studies, the pooled crude incidence rate of maternal stroke was 30.0 per 100,000 pregnancies [71], which is nearly three times that of non-pregnant women of similar reproductive age. According to recent trend studies, the incidence rate of maternal stroke has unchanged [72] or even increased [73].
The risk of maternal stroke is higher in the peripartum and postpartum periods than in the antepartum period. A population-based cohort study from UK found that the risk of stroke was slightly lower in the antepartum period but 9-fold higher in the peripartum period (defined as 2 days prior to 1 day after delivery in this study) and 3-fold higher in the first 6 weeks postpartum compared to the non-pregnant period [74]. In this study, the risks of ischemic and hemorrhagic stroke were both increased during the high-risk period [74]. Similarly, a recent study showed an increased risk of cerebral venous thrombosis (CVT) during the postpartum period; this risk was the highest during the first 6 weeks postpartum (adjusted OR [95% CI], 18.7 [8.3–41.9]), but there was no significantly increased risk during the antepartum period (adjusted OR [95% CI], 1.2 [0.6–2.3]) [75].
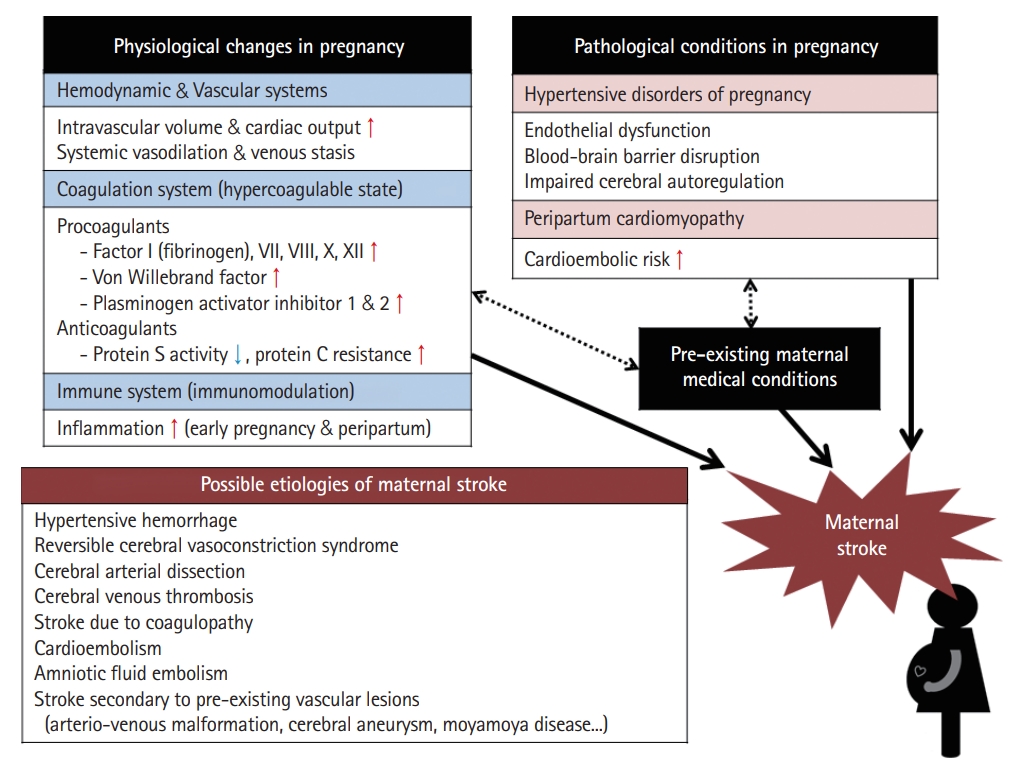
Physiological changes during pregnancy affecting stroke occurrence (Figure 2)
Various physiological changes occur during pregnancy. The three major changes associated with stroke are as follows: changes in the (1) hemodynamic/vascular system, (2) coagulation system, and (3) immune system.
Blood volume and cardiac output usually increase by ≈45% compared with pre-pregnancy levels to meet the increased metabolic demands of the mother and fetus [76]. Cardiac output peaks during labor and immediately after delivery, with increases of 60%–80% above levels seen before the onset of labor [76]. Physiological anemia occurs because plasma volume expansion is greater than the increase in red blood cells through erythropoiesis [76]. The increase in intravascular volume and cardiac output during pregnancy may worsen the condition of patients with pre-existing heart disease, contributing to increased risk of cardioembolic events including stroke [77]. These hemodynamic changes can also lead to increased hemodynamic stress on the arterial wall, and although controversial, it may affect the enlargement and increased rupture risk of arteriovenous malformations (AVMs) and cerebral aneurysms [78,79] or the development of dissection [80]. The major vascular change during pregnancy is vasodilation of the systemic vasculature, which causes a decrease in systemic vascular resistance and promotes venous stasis [76]. Venous stasis is exacerbated by compression of the inferior vena cava by the gravid uterus and decreased physical activity during late pregnancy and puerperium.
During pregnancy and the postpartum period, a hypercoagulable state is induced with a 4- to 10-fold increased thrombotic risk [81]. The hypercoagulable state results from an increase in procoagulant activity and a decrease in physiological anticoagulants. During pregnancy, the concentrations of coagulation factors VII, VIII, X, and XII and von Willebrand factor increase significantly accompanied by a pronounced increase in fibrinogen levels [81,82]. Physiological anticoagulation is reduced by a significant reduction in protein S activity and by acquired activated protein C resistance [81]. Fibrinolytic activity is also reduced during pregnancy due to increasing levels of plasminogen activator inhibitor-1 and placenta-derived plasminogen activator inhibitor type 2 [81,82]. In women with pre-existing hematologic disease, such as antiphospholipid syndrome and sickle cell disease, the risk of thrombotic events including stroke is even higher [83].
Pregnancy is considered a period of immunomodulation with greater inflammation in early and late pregnancy and lesser inflammation in mid-pregnancy [84]. Immune cells and their byproducts including many cytokines and growth factors play an important role in the implantation and establishment of the placenta in early pregnancy and in the induction of labor and delivery in the peripartum period [85]. However, an exaggerated inflammatory response due to dysregulation of this immunomodulation may lead to endothelial dysfunction, which is known to cause preterm birth and preeclampsia [86] and may increase stroke risk [87].
Pathological conditions in pregnancy affecting stroke occurrence (Figure 2)
• Hypertensive disorders of pregnancy: Hypertensive disorders of pregnancy (HDPs) are common medical complications of pregnancy. HTN in pregnancy can be chronic (predating pregnancy or diagnosed before 20 weeks of pregnancy) or de novo (either preeclampsia or gestational HTN) [88]. HDPs are classically classified into four types (each prevalence %) as follows: (1) preeclampsia (5%–7%), (2) gestational HTN (6%–7%), (3) chronic HTN (1%–5%), and (4) chronic HTN with superimposed preeclampsia (0.2%–1%) [89]. In a US nationwide cross-sectional study, women with HDPs were 5.2 times more likely to have a maternal stroke than those without [90]. HDPs are related not only to maternal stroke but also to future stroke [91]. The pathogenesis of HDPs remains unclear. One of the proposed mechanisms of preeclampsia is that reduced placental perfusion due to failed remodeling of the maternal vessels supplying the intervillous space leads to oxidative stress [92,93]. Then, oxidative stress stimulates activation of an excessive systemic inflammatory response, which causes endothelial dysfunction and vasoconstriction, leading to systemic HTN and end-organ hypoperfusion [92,93]. Endothelial dysfunction, blood-brain barrier disruption, and impaired cerebral autoregulation may play a central role in the cerebrovascular complications seen in preeclampsia [94].
• Peripartum cardiomyopathy: Peripartum cardiomyopathy (PPCM) is characterized by left ventricular (LV) systolic dysfunction and symptoms of heart failure (HF) that occur between the late stages of pregnancy and the early postpartum period without other cause of LV dysfunction. In a meta-analysis, patients with PPCM had a much higher prevalence of preeclampsia (22%) more than four times the average global rate expected in the general population (5%) [95]. In addition to preeclampsia, multiple gestations and multiparity are risk factors for the development of PPCM [95]. The pathogenesis of PPCM is poorly understood but may be related to a genetic predisposition and the toxic roles of profound pregnancy hormonal fluctuations during the third trimester and at birth that result in a disrupted maternal cardiovascular and metabolic homeostasis [96].
The majority of PPCM cases occur in the postpartum period, and most women present with signs and symptoms of HF [97]. In a multinational registry including over 700 women with PPCM from 49 countries, symptom-onset occurred most often within one month of delivery (44%), and 67% had an LV ejection fraction ≤35% at diagnosis [98]. The incidence of thromboembolic events was 7%, and stroke occurred in 3% by six months. The six-month mortality rate was 6%, and myocardial recovery occurred only in 46% [98].
Etiology of maternal stroke (Figure 2)
Hemorrhagic strokes account for almost 60% of strokes in pregnancy and the postpartum period, which is much higher than the rate in the general population [99]. Uncontrolled HTN and coagulopathy such as disseminated intravascular coagulation or HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome can cause hemorrhagic maternal stroke. In a recent cohort study, of the women who experienced ICH during pregnancy and the postpartum period, approximately one-third (35.3%) had preeclampsia/eclampsia, and 9.2% had coagulopathy [100]. Hemorrhagic maternal stroke can occur secondary to rupture of pre-existing vascular lesions such as AVMs and cerebral aneurysms [78,79].
Various vasculopathies can cause maternal stroke. Reversible cerebral vasoconstriction syndrome (RCVS) is a common etiology of maternal stroke, and most commonly occurs postpartum [101]. RCVS causes vasospasm of the cerebral arteries and can be associated with both hemorrhagic and ischemic strokes. Cerebral arterial dissection is also an important etiology of maternal stroke. In a recent case-control study, pregnancy was associated with a higher risk of cervical artery dissection (incidence rate ratio [95% CI], 2.2 [1.3–3.5]), and this risk was particularly high during the postpartum period [80]. Hemodynamic stress on arterial wall caused by increase in intravascular volume and cardiac output, altered arterial elasticity and stiffness due to hormonal changes, and vessel wall damage induced by Valsalva maneuvers during labor are suggested as possible mechanisms of pregnancy-related dissection, but it is still uncertain [80]. Moyamoya disease is a non-inflammatory vasculopathy that can be challenging to manage in pregnancy because there is a risk of both hemorrhagic and ischemic stroke [102].
Cardioembolism is an important etiology of maternal strokes, as well. For example, pregnant women with pre-existing heart disease such as congenital heart disease could be at risk of cardioembolic stroke [103]. In addition to pre-existing cardiac conditions, PPCM is associated with maternal stroke [98]. Rarely, maternal strokes due to endocarditis [104] and amniotic fluid embolism [105] have also been reported.
Hypercoagulability in pregnancy and postpartum period can promote both arterial and venous thrombosis. CVT is a common etiology of maternal stroke and occurs mainly during the postpartum period [75,101]. Propagation of thrombosis causes venous congestion, leading to venous infarct, hemorrhage, and increased intracranial pressure. Considering the five-times higher risk of deep vein thrombosis in pregnancy and the postpartum period [106], paradoxical embolism through a PFO can cause embolic stroke.
Adverse pregnancy outcomes and long-term risk of stroke
HDPs are well-known adverse pregnancy outcomes that increase long-term stroke risk [91,107,108]. In a meta-analysis of preeclampsia and future stroke, preeclampsia was associated with a ≈2-fold increased stroke risk [108]. A recent Taiwanese nationwide study with an overall follow-up period of 17 years investigated future stroke risk by stratification according to follow-up time and HDP subtype [91]. Women with HDPs had an adjusted HR (95% CI) of 1.71 (1.46–2.00) for stroke: 1.60 (1.35–1.89) and 2.98 (2.13–4.18) for ischemic and hemorrhagic stroke, respectively. Although the risks of both ischemic and hemorrhagic stroke persisted for up to the overall follow-up period, their risk time trends were different. The risk of ischemic stroke peaked around one to three years after childbirth with an HR of 2.14 (95% CI, 1.36–3.38), while that of hemorrhagic stroke increased after five years and kept increasing with time with an HR of 4.64 (95% CI, 2.47–8.73) after 10–15 years of childbirth. Regarding the HDP subtype, stroke risks were higher in women with HDP with preeclampsia than in women with HDP without preeclampsia. In detail, among the four HDP subtypes, chronic HTN with superimposed preeclampsia had the highest stroke risk followed by preeclampsia-eclampsia, gestational HTN, and chronic HTN with HRs (95% CI) of 3.86 (1.91–7.82), 2.00 (1.63–2.45), 1.68 (1.13–2.52), and 1.27 (0.97–1.68), respectively.
In a recent systematic review, other adverse pregnancy outcomes including gestational diabetes (RR [95% CI], 1.21 [1.05–1.40]) and preterm birth (HR [95% CI], 1.71 [1.53–1.91]) were associated with long-term stroke risk [109]. A recent Swedish national cohort of >2 million women found that preterm delivery was associated with higher future risks of both hemorrhagic and ischemic stroke up to at least 40 years even after adjusting for established risk factors with cosibling factors to account for shared genetic and environmental factors within families [110].
Exogenous estrogen
Use of oral contraceptive pills (OCPs) is associated with an increased risk of stroke [111-113]. In previous meta-analyses, women with current OCP use were at a ≈2.5-fold increased risk of ischemic stroke [111] and a ≈1.4-fold increased risk of hemorrhagic stroke [112]. The risk of stroke increases as the estrogen dose increases [111,113], but the progestin-only pill is not associated with increased risk of ischemic stroke [111,114]. The stroke risk in women with current OCP use is further increased by additional risk factors such as migraine, HTN, and current smoking [111,112]. According to a recent study, the stroke risk in women with OCP use was particularly high during the first year of OCP use, possibly due to immediate changes in hemostatic balance [115].
Oral menopausal hormone replacement therapy (HRT) also increases the risk of ischemic stroke [116,117]. In the two Women’s Health Initiative hormone therapy trials, the stroke risk was increased with both estrogen alone and estrogen plus progestin therapies and the risk was not evident for hemorrhagic stroke but was for ischemic stroke [117]. However, in a recent study, HRT was also associated with an increased event rate of SAH [115]. In this study, similarly with the result of OCP use, the stroke risk was especially high during the first year of HRT use (HR [95% CI], 2.12 [1.66–2.70]) and remained increased during remaining years of use (HR [95% CI], 1.18 [1.05–1.31]). Regarding the initiation of HRT, the “timing hypothesis” has been suggested that age and time since menopause influence the relationship between HRT and CVD [118]. Starting HRT might be more harmful when started more than 10 years after the onset of menopause or in women older than 60 years of age [119]. Observational studies suggest that the use of transdermal therapy containing low doses of estrogen has a lower stroke risk than oral HRT [119,120].
Lifetime endogenous estrogen exposure
The reproductive lifespan (defined as the time from menarche to menopause), which reflects endogenous estrogen exposure, is closely related to stroke risk [121]. In a large pooled analysis, women with a reproductive life span less than 30 years had a 75% higher risk of stroke than those with a reproductive life span of 36–38 years [121]. Compared with women with menarche at age 13 years, those with early menarche (≤10 years) and late menarche (≥16 years) had 27% and 25% increased risk of stroke, respectively. Compared with women aged 50–51 years at menopause, those with premature menopause (<40 years) and early menopause (40–44 years) had 98% and 49% higher risk of stroke, respectively [121]. In addition to natural menopause, surgical menopause by oophorectomy (with or without hysterectomy) is also associated with a higher risk of stroke [122,123]. Conflicting results have been reported regarding hysterectomy. In a previous meta-analysis, hysterectomy appeared to reduce the risk of stroke [122], but there are also contradictory results [123]. In a recent prospective study of almost 300,000 Chinese women, women who underwent hysterectomy alone and hysterectomy with bilateral oophorectomy had an ≈6% and ≈20% higher ischemic stroke risk, respectively, than those who did not undergo surgery, and these RRs were more extreme for those who were younger at the time of surgery [123].
Stroke outcomes (Figure 3)
Regarding stroke mortality, contradictory results have been shown depending on adjustments for confounders [4,124]. In the INSTRUCT (International Stroke Outcomes Study), a meta-analysis of individual participant data from 13 population-based incidence studies, the crude mortality rate ratio (MRR) was higher for women than men at 1 year (MRR [95% CI], 1.35 [1.24–1.47]) and 5 years (MRR [95% CI], 1.24 [1.12–1.38]) [124]. However, these results were reversed after adjusting for confounders including age, pre-stroke functional limitations, stroke severity, and history of AF (1-year MRR [95% CI], 0.81 [0.72–0.92] and 5-year MRR [95% CI], 0.76 [0.65–0.89]); this indicates that women’s higher mortality after stroke is attributable to these confounders (advanced age, greater stroke severity, worse pre-stroke status, and higher prevalence of AF) [124]. Similarly, in a recent prospective 25-year follow-up study from the UK, women had a 9% lower risk of death than men after full adjustment, while they had a 30% higher risk of death than men in an unadjusted analysis [4]. In a pooled analysis of individual participant data from five acute stroke randomized controls trials, after multivariable adjustments, women with ischemic stroke had higher survival rates at 3–6 months than men; however, this higher survival rate in women was not significant for ICH [125].
Women have poorer functional recovery and lower quality of life (QOL) than men after stroke. This finding has been consistently reported in many studies even after adjusting for several confounders [4,10,125-128]. Advanced age, more severe strokes, poorer pre-stroke function, more comorbidities, less social support, and a higher likelihood of being a widow are factors considered to contribute to a less favorable outcome in women; however, the causes have yet to be fully elucidated because adjustment for these factors does not adequately explain the sex differences in functional recovery and QOL after stroke [4,10,125-128].
Post-stroke depression (PSD) is more common in women than in men [126,129]. A previous systematic review suggested that the prevalence of PSD is 78% higher among women than among men [129]. In a more recent systematic review, women showed a higher likelihood of PSD (OR range, 1.27–3.15 and HR, 3.52) or higher mean PSD symptoms in an unadjusted analysis [126]. Even after multivariable adjustment for age, stroke severity, and activity limitations, women were still more likely to have a significantly higher prevalence, incidence, or symptoms of depression than men [126]. In addition to depression, women appear to have worse outcome than men in anxiety [4].
Regarding post-stroke cognitive impairment, previous studies have shown conflicting results [126]. In a recent population-based study examining sex differences in cognitive outcomes at 90 days after stroke among first-ever stroke patients, women had significantly lower levels of cognitive function and a higher unadjusted prevalence of dementia than men (35.6% vs. 27.6%) [130]. The differences were fully attenuated after adjustment for covariates, indicating that the major contributing factors to the worse post-stroke cognitive outcomes in women were a higher prevalence of being widowed, older age at stroke onset, worse pre-stroke functional and cognitive status, and lower educational attainment. Another recent cohort study of community-dwelling adults in the US found that women experience more post-stroke cognitive deficits even after controlling for pre-stroke cognitive measures, particularly during the early post-stroke period [131].
Conclusion
Women face a disproportionate burden of stroke. Women are more likely to have a stroke in their lifetime and have poorer post-stroke outcomes. There are a number of sex differences in the impact of conventional risk factors on stroke, which indicates that the optimal management of stroke risk factors may differ between men and women. For better understanding of this, the problem of underrepresentation of women in clinical trials [132] must be improved and well-designed studies powered to detect sex-based differences are required. Further research into the underlying mechanisms responsible for these sex differences is also warranted. Women have unique stroke risk factors such as pregnancy, endogenous hormone levels, and exogenous hormone therapy. These women-specific risk factors should be considered in optimal risk assessment and tailored preventive strategies for women.
Notes
Disclosure
The authors have no financial conflicts of interest