 |
 |
- Search
| J Stroke > Volume 25(2); 2023 > Article |
|
Abstract
Background and Purpose
To evaluate whether the thrombus enhancement sign (TES) can be used to differentiate embolic large vessel occlusion (LVO) from in situ intracranial atherosclerotic stenosis (ICAS)-related LVO in the anterior circulation of patients with acute ischemic stroke (AIS).
Methods
Patients with LVO in the anterior circulation who underwent both non-contrast computed tomography (CT) and CT angiography and mechanical thrombectomy were retrospectively enrolled. Both embolic LVO (embo-LVO) and in situ ICAS-related LVO (ICAS-LVO) were confirmed by two neurointerventional radiologists after reviewing the medical and imaging data. TES was assessed to predict embo-LVO or ICAS-LVO. The associations between occlusion type and TES, along with clinical and interventional parameters, were investigated using logistic regression analysis and a receiver operating characteristic curve.
Results
A total of 288 patients with AIS were included and divided into an embo-LVO group (n=235) and an ICAS-LVO group (n=53). TES was identified in 205 (71.2%) patients and was more frequently observed in those with embo-LVO, with a sensitivity of 83.8%, specificity of 84.9%, and area under the curve (AUC) of 0.844. Multivariate analysis showed that TES (odds ratio [OR], 22.2; 95% confidence interval [CI], 9.4-53.8; P<0.001) and atrial fibrillation (OR, 6.6; 95% CI, 2.8-15.8; P<0.001) were independent predictors of embolic occlusion. A predictive model that included both TES and atrial fibrillation yielded a higher diagnostic ability for embo-LVO, with an AUC of 0.899.
Mechanical thrombectomy has become a standard approach for the treatment of patients with acute large vessel occlusion (LVO) stroke in the anterior circulation [1,2]. Traditional classification of stroke is based on the International Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification [3]. However, the etiology at the LVO site can differ, with embolism being the most common cause of acute ischemic stroke (AIS) [4]. Previous studies have reported several embolic sources, including cardiac, proximal atherosclerotic, and arterial dissection, as well as in situ intracranial atherosclerotic stenosis (ICAS) or dissection-related LVO [5]. Therefore, the TOAST classification is often inappropriate for the identification of pathogenesis at the site of LVO. The clinical presentation, risk factors, and demographic features of patients with LVO caused by embolism (embo-LVO) or ICAS-related LVO (ICAS-LVO) are the most important issues to be identified. Accurate identification of the specific causes of occlusion affects strategic planning for perfusion therapy and outcome prediction [6]. While thrombectomy is more effective for embo-LVO [7], re-occlusion and residual stenosis are often encountered after thrombectomy for ICAS-LVO, which frequently requires rescue treatment with balloon angioplasty or stents [8]. Moreover, until now, no sensitive imaging biomarkers have been used to accurately distinguish between embolic and ICAS-related LVO.
Computed tomography angiography (CTA) is routinely used to identify arterial occlusion when patients are suspected of having acute LVO ischemic stroke [9]. Imaging features, such as hyperdense artery sign and thrombus perviousness, have been suggested as potential indicators of thrombus composition and origin, but the diagnostic accuracy of these image biomarkers ranges only from 50% to 70% [10,11]. Recently, we reported the thrombus enhancement sign (TES) on thin-slab maximum intensity projection (TS-MIP) image reconstruction of computed tomography (CT) angiograms as a new imaging biomarker to predict stroke etiology [12,13]. The proportion of TES-positive patients in cardioembolic and cryptogenic strokes was 92%, and most cryptogenic strokes were considered as cardiac. Meanwhile, only 29% ICAS-LVO stroke patients were TES-positive. Thus, we believe that the TES might be a more accurate predictor of pathogenesis at the occlusion site than the TOAST classification. The potential mechanisms of TES sign formation reflect the penetration ability of the contrast agent, which largely depends on the existence of potential gaps between the thrombus and the arterial wall or within the thrombus. For embolic lesions, there are many potential gaps owing to the large thrombus load and failure of adaptive deformation of the thrombus in the vessel lumen. In contrast, the TES rarely appears in ICAS-LVO because of the small thrombus load. Therefore, we believe that the TES sign may be more accurate in distinguishing whether LVO is caused by an embolism or ICAS. In this study, we explored the association between TES and different pathogenesis-related LVO, along with their clinical parameters.
This single-center retrospective study was conducted in accordance with the recommendations of the Declaration of Helsinki and was approved by the institutional research ethics committee of Shanghai Sixth PeopleŌĆÖs Hospital affiliated to Shanghai Jiao Tong University School of Medicine (No 2018-002-1). Informed consent was obtained from patients or their relatives. Patients with acute LVO stroke involving the distal internal carotid artery (ICA) or the proximal middle cerebral artery (MCA) between January 2018 and September 2021 were included. These patients underwent non-contrast CT (NCCT) and CTA before undergoing endovascular reperfusion therapy. Patients were excluded if NCCT or CTA were not available or if the image quality was insufficient.
NCCT and CTA were performed using a 64-section CT scanner (Brilliance 64; Philips Healthcare, Amsterdam, the Netherlands) with the following parameters: tube voltage, 120 kV; tube current, 333 mA; and rotation speed, 0.75 s, or using a 640-section CT scanner (United Imaging Healthcare, Shanghai, China) with the following parameters: tube voltage, 120 kV; tube current, 300 mA; rotation speed, 0.5 s. An intravenous contrast agent (60-100 mL of iopromide, 370 mg iodine per mL) (Ultravist 370; Bayer, Leverkusen, Germany) was injected at a flow rate of 4 mL/s. Scanning was started when the CT attenuation in the ascending aorta reached 120 Hounsfield units (HU). Volume rendering and full-slab MIP image processing were performed at a multimodality workstation (Philips Healthcare) using 0.67-mm axial sections and were reconstructed at an increment of 0.8 mm. The acquired axial-slice CT angiograms were transferred to a dedicated workstation for TS-MIP construction (SyngoXWP; Simens Healthcare, Erlangen, Germany). After the 0.67-mm axial images were loaded into the InSpace section at the workstation, the digital subtraction angiography layout application (Siemens Healthcare) was used, and coronal MIP was applied for image reconstruction using a 10-mm-thick slab.
Angiography and thrombectomy were performed using a biplane digital angiography unit (Artis Zee; Simens Medical Solutions, Erlangen, Germany). The SWIM technique (Solitaire stent retriever in combination with the intracranial support catheter aspiration for mechanical thrombectomy; ev3 Neurovascular, Medtronic, Minneapolis, MN, USA) was used as the first-line technique [14]. After a 90 cm sheath (Terumo, Tokyo, Japan) was introduced into the cervical segment of the ICA on the affected side, an aspiration catheter (ACE68 or ACE60; Penumbra, Alameda, CA,USA) was advanced into the distal ICA with a microcatheter over a microwire. Next, the microcatheter was placed distal to the thrombus, and a stent retriever (Solitaire, ev3 Neurovascular) was deployed through the thrombus according to the diameter of the occluded artery (usually 6├Ś30 mm for the ICA terminus or proximal M1 and 4├Ś20 mm for the distal M1 or M2); 3-5 min were allowed for clot integration. Using the stent retriever as an anchor, the aspiration catheter was maneuvered up to the proximal side of the stent to simultaneously increase the effect of aspiration. The stent retriever was withdrawn from the aspiration catheter and was completely removed from the system. Subsequently, the aspiration catheter was removed under continuous aspiration and proximal flow arrest to ensure aspiration of the potential microfragments of the thrombus. In cases of failure of the SWIM technique, catheter aspiration or double-stent thrombectomy was performed. If ICAS-LVO was suspected in situ during thrombectomy and re-occlusion occurred, salvage balloon dilation or stent insertion was performed. Antegrade reperfusion was assessed using the modified Thrombolysis in Cerebral Infarction (mTICI) grade. Successful reperfusion was defined as mTICI 2b-3 after endovascular treatment.
Thrombus burden was assessed using the clot burden score (CBS) according to the method by Puetz et al. [15]. A score of 10 on the CBS indicates that no occlusion is present, whereas a score of 0 implies complete occlusion. TES was defined as the contrast agent penetrating partially or completely into the thrombus or the space between the thrombus and arterial wall to make the thrombus visible or discriminable on CTA imaging according to our previous experience [12]. CBS and TES were determined by two interventional neuroradiologists who were blinded to the clinical information, and the angiographic or procedural results were determined after inter-reader agreement were reached. In cases of disagreement between the readers, a third neuroradiologist joined, and a consensus was reached by the three readers in an additional joint review session that took place less than 2 weeks after the individual reading sessions.
Embo-LVO and ICAS-LVO were determined by an interventional neuroradiologist based on clinical and imaging data, except for CT angiograms before reperfusion therapy. Occlusion was considered an embo-LVO if (1) no focal stenosis was indicated after immediate thrombectomy; (2) postoperative CTA or magnetic resonance angiography (MRA) revealed no stenosis of the affected artery within 7 days; or (3) no significant stenosis was found at the affected artery on CTA or MRA within 3 months before the onset of stroke. Underlying in situ ICAS-related LVO was considered if (1) significant focal stenosis (Ōēź70%) was observed at the site of occlusion after first-line SWIM or at final angiography, or (2) fixed stenosis Ōēź50% was observed with evident impaired perfusion or evidence of re-occlusion after repeated stent retriever thrombectomy [16,17].
Data are presented as mean┬▒standard deviation, median (interquartile range), or number and frequency, as appropriate. The Kolmogorov-Smirnov test was used to assess the assumption of a normal distribution. Comparisons between the embo-LVO and ICAS-LVO groups were conducted using a Žć2 test for categorical variables and Student t-test or Mann-Whitney U test for noncategorical variables with or without normal distribution, respectively. Comparisons between the patients with and without TES were also performed. The association between embolism-related occlusion and TES, along with the clinical and interventional parameters, was investigated using univariate and multivariate logistic analyses. Parameters with P values <0.1 in univariate analysis were included in a final multivariate logistic regression model. Independent parameters were assessed using receiver operating characteristic (ROC) curve analyses with sensitivity, specificity, predictive values, accuracy, and area under the curve (AUC) for predicting embolism-related occlusion. The inter-reader agreement for TES was determined using ╬║ values with a grading system as follows: perfect, ╬║=0.81-1.00; substantial, ╬║=0.61-0.80; moderate, ╬║=0.41-0.60; and poor, ╬║Ōēż0.40. All analyses were conducted using the open-source R software (version 4.0.3; R Foundation for Statistical Computing, Vienna, Austria). A two-sided P<0.05 was considered statistically significant.
Between January 2018 and September 2021, 376 consecutive patients underwent mechanical thrombectomy at our comprehensive stroke center. After excluding patients with posterior circulation strokes (n=48), those who did not undergo pre-procedure CTA or had a suboptimal scan (n=32), and patients with persistent occlusion making embo-LVO or ICAS-LVO undetermined (n=8), a total of 288 patients qualified for this study (Figure 1).
Among the included patients, TES was detected in 74.7% (215/288) of patients by reader 1 and in 70.1% (202/288) of patients by reader 2. After consensus was reached on disagreements, TES was observed in 71.2% of the enrolled patients (205/288). The two readers had substantial to complete interobserver agreement with regards to measuring the presence of TES (╬║=0.825; 95% confidence interval [CI], 0.635-0.942) and CBS (╬║=0.786; 95% CI, 0.568-0.915). The results of comparisons between the patients with positive TES (n=205; mean age, 71.9┬▒11.7 years; age range, 37-90 years; 121 men) and those with negative TES (n=83; mean age, 71.0┬▒11.4 years; age range, 35-88 years; 43 men) are shown in Table 1. Patients with positive TES had significantly higher CBS (6.6┬▒1.1) than those with negative TES (6.2┬▒1.2, P=0.007). Atrial fibrillation was more frequent in patients with positive TES (67.3%, 138 of 205) than in patients with negative TES (33.7%, 28 of 83) (P<0.001). Positive TES was found in 96.1% (197/205) of embo-LVO patients, whereas negative TES was found in 54.2% (45/83) of ICAS-LVO patients (P<0.001) (Figures 2 and 3).
Two hundred and thirty-five patients (81.6%) were included in the embo-LVO group. Among them, no stenosis of the affected artery was confirmed in 75.7% (178/235) patients by immediate angiography after thrombectomy, 67.7% (159/235) patients by postoperative CTA or MRA within 7 days, and in 13.6% (32/235) patients by preoperative CTA or MRA within 3 months. Fifty-three patients (18.4 %) were included in the ICAS-LVO group. The median degree of stenosis was 63% (interquartile range 52%-75%). The results of the comparison between the embo-LVO and ICAS-LVO groups are presented in Table 2. The mean baseline National Institutes of Health Stroke Scale (NIHSS) score was significantly higher in the embo-LVO group compared with the ICAS-LVO group (16.4┬▒5.3 vs. 14.8┬▒4.6, P=0.042). Atrial fibrillation was found in 66.8% (157/235) of patients in the embo-LVO group and in 17.0% (9/53) of patients in the ICAS-LVO group (P<0.001). There were no significant differences in other risk factors between the two groups.
TES was visualized in 83.8% (197/235) of patients in the embo-LVO group and in 15.1% (8/53) of patients in the ICAS-LVO group (P<0.001). In univariate logistic analysis, TES, baseline NIHSS score, and atrial fibrillation had significant effects on embo-LVO. Multivariate analysis showed that TES (odds ratio [OR], 22.2; 95% CI, 9.4-53.8; P<0.001) and atrial fibrillation (OR, 6.6; 95% CI, 2.8-15.8; P<0.001) were independent predictors of embo-LVOs when adjusting for baseline NIHSS (Table 3). In the prediction of embo-LVOs, TES had a sensitivity of 83.8% (95% CI, 76.5%-85.8%), a specificity of 84.9% (95% CI, 71.8%-92.8%), a positive predictive value of 96.1% (95% CI, 92.2%-98.2%), a negative predictive value of 54.2% (95% CI, 43.0%-65.1%), an accuracy of 84.0% (95% CI, 79.3%-87.8%), and an AUC of 0.844 (95% CI, 0.782-0.906) (Table 4). The predicted probabilities of the multivariate logistic regression model were further tested using ROC analysis. The AUC was 0.899 (95% CI, 0.854-0.943, P<0.001).
Our current study indicated that (1) TES was significantly associated with embo-LVOs; (2) a positive TES could be used for the diagnosis of embo-LVOs with high sensitivity and specificity; and (3) TES was an independent predictor for embo-LVOs. These findings indicate that TES could be used as an imaging biomarker for the identification of specific causes of LVO, which may be very helpful in making therapeutic plans during endovascular reperfusion treatment.
The accurate identification of embolic or in situ ICAS-related LVO is an important clinical issue in stroke treatment. Achieving a high first-pass effect (FPE) rate after thrombectomy is critical for improving the prognosis of AIS patients. Compared with ICAS, embo-LVO has a different pathogeny and reperfusion response to mechanical thrombectomy. Recanalization of embo-LVOs often indicates a shorter procedural time, higher incidence of FPE, lower number of passes, and a higher rate of final successful recanalization [18]. For ICAS lesions, if a significant in situ underlying stenosis is present at the occlusion artery, the FPE rate is often lower, and recanalization may not be achieved sufficiently even after repeated thrombectomy [19]. Although the optimal treatment for underlying ICAS in patients presenting with hyperacute stroke remains unknown, achieving successful reperfusion immediately after first-line thrombectomy was more frequent in the stent retriever group than in the catheter aspiration group [20]. In addition, a rescue therapy strategy of balloon dilation or stenting was often required.
Recent studies have identified imaging predictors that may help differentiate the two occlusion subtypes, such as thrombus imaging, diffusion-weighted imaging, vessel calcification, and collateral circulation [21,22]. Meanwhile, recent advances have found susceptibility vessel signs using susceptibility-based magnetic resonance imaging (MRI), which can be used to discriminate embo-LVOs and ICAS-LVOs [23,24]. However, this imaging sign showed poor specificity and accuracy [25]. Some specific imaging biomarkers, such as the hyperdense artery sign, meniscus sign, truncal-type thrombus occlusion, branching-site thrombus occlusion, stent deployment status sign, angiographic bypass effect, and clot protrusion sign, have been reported for the direct prediction of embo-LVOs [26-29]. Meanwhile, other imaging signs are believed to be closely related to ICAS. The microcatheter first-pass sign, referred to as forward blood flow restoration after microcatheter pass and withdrawal through the occlusive segment, was observed in 90.9% of patients with ICAS and 12.8% of patients with intracranial embolism with high sensitivity, specificity, positive predictive value, and accuracy [30]. Triad signs after thrombectomy and recanalization include (1) residual fixed stenosis following mechanical thrombectomy that measures >50%; (2) mild-to-moderate stenosis with re-occlusion on follow-up angiography; or (3) evidence of distal hypoperfusion and ruling out other pathologies, such as dissection, also help to determine ICAS-LVOs [31]. However, currently reported imaging signs have limitations for their clinical application. The reason for this is that most of these signs are obtained during catheterized angiography or thrombectomy instead of CT scans. MRI scanning to identify these imaging signs often requires extra time and better cooperation from patients to keep their heads motionless throughout the entire examination period, which limits its clinical application. Thus, it is necessary to identify more reliable imaging signs to differentiate embolisms from ICAS-related occlusions.
To the best of our knowledge, this is the first study to demonstrate that TES can be used to predict embo-LVO. In line with previous results by Wei et al. [12], TES is an imaging sign created by the contrast agent penetrating the space between the thrombus and arterial wall or within the thrombus. TES and previously reported residual flow in clots share many similarities because they both describe the status of contrast penetration within the occluded vessel [32]. However, we found some differences between the two. First, the residual flow was positive in 17.1% [33] and 36% [32] of patients when assessed on 24-mm and 3-mm-thick CTA-MIP images, respectively, whereas we assessed TES on 10-mm CTA-MIP images. We believe that 10 mm is the optimal thickness to display thrombus enhancement that is denser than the surrounding brain parenchyma with a higher sensitivity (71.2% of the patients in the present study). Second, in a previous study, residual flow was divided into three grades to assess patient response to intravenous thrombolysis, whereas in the era of thrombectomy, dichotomous positive and negative classification may become more clinically applicable for the prediction of thrombus components, identifying stroke source, and ICAS differentiation. We believe that the possible mechanisms that are more likely to cause TES are that (1) normal vessel lumen levels at the embolic site facilitate the infiltration of more contrast agents; (2) embo-LVO usually has a large thrombus load; therefore, after thrombus embolization, the irregular space inside the thrombus or between the thrombus and the arterial wall increases the possibility of contrast penetration; and (3) the probability of infiltration of the contrast agent is higher for hard or fiber-rich thrombi. In contrast, in situ ICAS has a smaller lumen and thrombus load, which reduces the contrast agent penetration. The thrombus formed at the arteriosclerotic site mainly consisted of a fibrin network superimposed on the underlying platelet aggregates, making it even more difficult for the contrast agent to penetrate. Therefore, TES can be used as a reliable imaging sign to differentiate embo-LVO from ICAS-LVO in patients with AIS.
Interestingly, eight of the 53 patients with underlying large artery arteriosclerosis in this study showed positive TES. We found that all of these patients suffered from clot embolism of proximal artery atherosclerosis origin. Even with a discrepancy in the texture and composition of the clot, the mechanism of TES formation from distal embolism due to proximal large artery arteriosclerosis was similar to that of cardiac or other source-derived embolisms. Despite the different sources of embolic clots, identification of TES before endovascular therapy can aid interventionists to determine appropriate therapeutic strategies [8]. TES derived from routine CTA imaging data can be assessed without time delay to ensure wide availability. Although TES showed high accuracy in identifying embo-LVOs with strong inter-reader agreement, the negative predictive value was moderate, indicating that the absence of TES in patients with ICAS should not be used to argue against more extensive workup and monitoring for the detection of potential intracranial atherosclerotic plaques, if clinically indicated. Other clinical factors, such as a history of atrial fibrillation and baseline NIHSS score, have been proposed as biomarkers related to embo-LVOs. In the present study, patients in the embo-LVO group had a significantly higher frequency of atrial fibrillation. This is consistent with previous studies [34,35]. Persistent atrial fibrillation is known to be the leading cause of cardioembolism [36]. Therefore, patients with atrial fibrillation are more vulnerable to embo-LVO. The baseline NIHSS score was significantly lower in the ICAS-LVO group, possibly because of ischemic preconditioning and better collateral circulation. In addition, there was no significant difference in angiography or functional outcomes between embo-LVOs and ICAS-LVOs, which was consistent with other studies [7]. Our results suggested that the use of rescue therapy for ICAS-LVOs was safe and effective and did not lead to a poor functional outcome.
This study had several limitations. First, it was a single-center retrospective study with inherent selection bias. Second, we did not evaluate the collateral status in this analysis, as collateral flow, whether residual antegrade or retrograde, is important for identifying TES. In rare cases, when the proximal end of the thrombus (new thrombus) is tightly attached to the vessel, the contrast agent can still retrogradely penetrate the thrombus from its distal end when sufficient backward flow of the collateral vessel is achieved. Finally, the identification of embo-LVOs and ICAS-LVOs after thrombectomy depended mainly on the imaging characteristics of the local lesion during the procedure. It is difficult to determine whether embolism or ICAS exists in some patients because patients with persistent occlusion were excluded; this should be taken into consideration.
In conclusion, TES is a sensitive and specific marker with high predictive value for the identification of embolism-related occlusion stroke, offering guidance regarding subsequent endovascular revascularization therapy. Further prospective studies with larger sample sizes are warranted to confirm our findings.
Notes
Funding statement
This study was supported by Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (No. 20152528), Shanghai Jiao Tong University ŌĆ£Medical and ResearchŌĆØ Program (ZH2018ZDA19), Suzhou Science and Technology Development Program (SYSD2020033), and Suzhou Technology Innovation Project of Medical and Health Science (sky2022026).
Author contribution
Conceptualization: YZ2, LW. Study design: YZ2. Methodology: YZ1, GH, JL. Data collection: YZ1, GH, JL, GM, DL, JW, JD. Investigation: YZ1, GH, JL, GM, DL, JW, JD. Statistical analysis: GH. WritingŌĆöoriginal draft: YZ1, GH, JL. WritingŌĆöreview & editing: YZ2, LW. Funding acquisition: YZ1, YZ2. Approval of final manuscript: all authors.
Figure┬Ā1.
Flow diagram of the current study. CTA, computed tomography angiography; embo-LVO, embolism-related large vessel occlusion; ICAS-LVO, intracranial atherosclerotic stenosis-related large vessel occlusion; TES, thrombus enhancement sign.
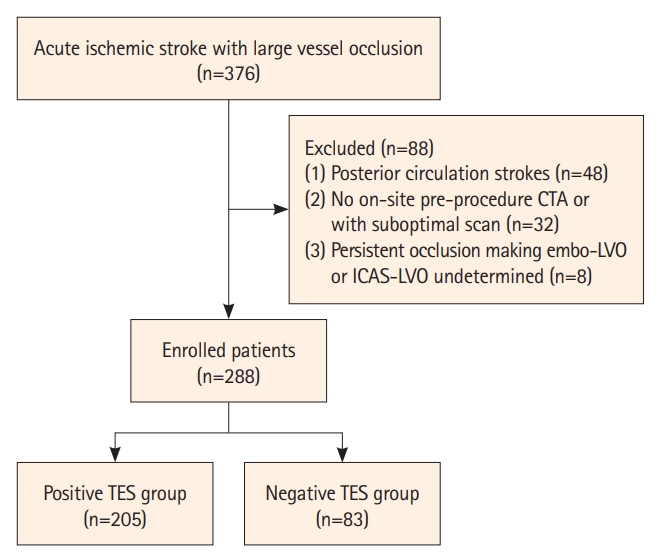
Figure┬Ā2.
Embolism-related LVO with a positive TES. (A) NCCT reveals HAS in the M1 segment of the right MCA (white arrow). (B) Original axial slice of CTA indicates a thrombus filling defect at the M1 segment of the right MCA (red arrow). (C and D) Volume rendering and full-slab MIP image reconstruction of CTA showing the occluded site (yellow arrow). (E) Anteroposterior angiogram shows the occlusion site at the M1 segment of the right MCA (yellow arrow). (F) Thin slab MIP image reconstruction of CTA shows the TES (red arrows) at right MCA. (G) Complete recanalization of the right MCA without residual stenosis after a single attempt of stent retrieval. (H) Schematic diagram of the pathogenesis of embolism-related LVO. LVO, large vessel occlusion; TES, thrombus enhancement sign; NCCT, non-contrast computed tomography; HAS, hyperdense artery sign; MCA, middle cerebral artery; CTA, computed tomography angiography; MIP, maximum intensity projection.
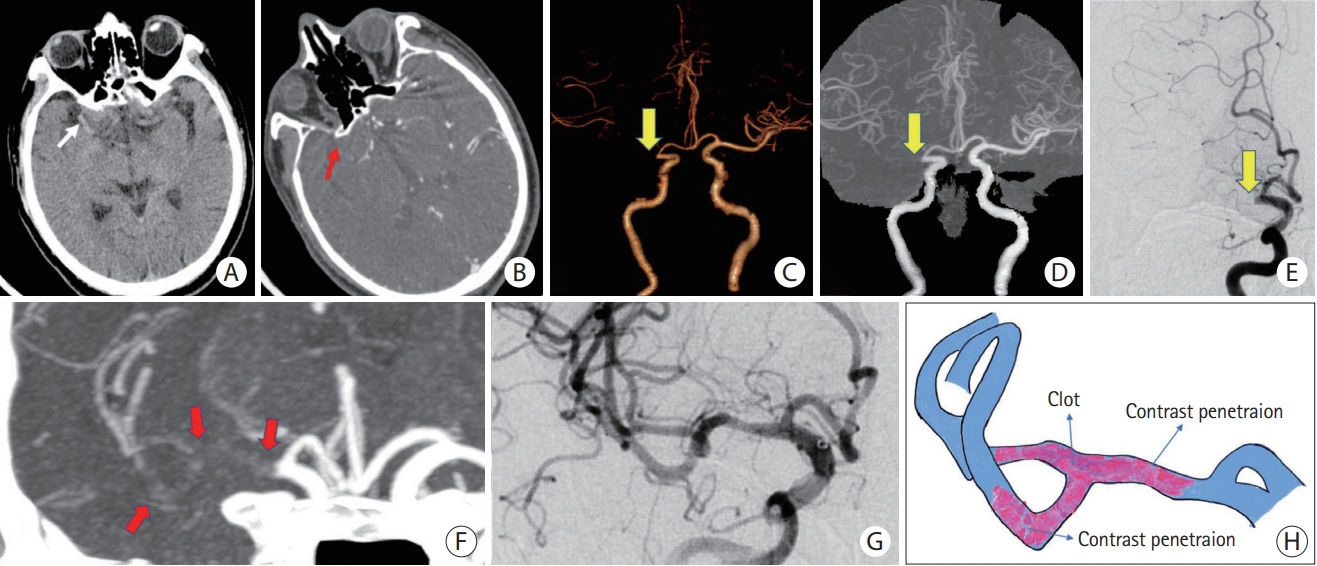
Figure┬Ā3.
ICAS-related LVO with a negative TES. (A) NCCT does not reveal HAS in the M1 segment of the left MCA (white arrow). (B) Original axial slice of CTA indicates thrombus filling defect at the M1 segment of the left MCA (red arrow). (C and D) Volume rendering and full-slab MIP image reconstruction of CTA showing the occluded site (yellow arrow). (E) Anteroposterior angiogram shows occlusion site at the M1 segment of the left MCA (yellow arrow). (F) Thin slab MIP image reconstruction of CTA shows a negative TES (red arrow) at the left MCA. (G) After attempting stent retrieval, rescued angioplasty and stenting was applied. Residual stenosis remains, but distal flow significantly improves. (H) Schematic diagram of the pathogenesis of ICAS-related LVO. ICAS, intracranial atherosclerotic stenosis; LVO, large vessel occlusion; TES, thrombus enhancement sign; NCCT, non-contrast computed tomography; HAS, hyperdense artery sign; MCA, middle cerebral artery; CTA, computed tomography angiography; MIP, maximum intensity projection.
Table┬Ā1.
Comparison between the positive and negative TES groups
| Characteristic | Positive TES (n=205) | Negative TES (n=83) | P |
|---|---|---|---|
| Age (yr) | 71.9┬▒11.7 | 71.0┬▒11.4 | 0.520 |
| Male sex | 121 (59.0) | 43 (51.8) | 0.294 |
| Baseline NIHSS | 16.5┬▒5.3 | 15.3┬▒5.0 | 0.074 |
| Baseline ASPECTS | 9.6┬▒0.8 | 9.7┬▒0.6 | 0.348 |
| Risk factors | |||
| Diabetes mellitus | 54 (26.3) | 18 (21.7) | 0.455 |
| Hypertension | 119 (58.0) | 52 (62.7) | 0.509 |
| Atrial fibrillation | 138 (67.3) | 28 (33.7) | <0.001* |
| Coronary artery disease | 65 (31.7) | 18 (21.7) | 0.114 |
| Dyslipidemia | 39 (19.0) | 19 (22.9) | 0.517 |
| Current smoking | 37 (18.0) | 12 (14.5) | 0.495 |
| Occlusion sites | 0.123 | ||
| ŌĆāMCA | 136 (66.3) | 63 (75.9) | |
| ŌĆāICA | 69 (33.7) | 20 (24.1) | |
| CBS | 6.6┬▒1.1 | 6.2┬▒1.2 | 0.007 |
| IVT | 86 (42.0) | 31 (37.3) | 0.509 |
| Onset to puncture (min) | 276.0┬▒155.5 | 253.6┬▒106.8 | 0.230 |
| Puncture to recanalization (min) | 55.6┬▒26.3 | 52.0┬▒23.8 | 0.278 |
| Procedure time (min) | 70.1┬▒27.4 | 93.6┬▒31.3 | <0.001* |
| Stroke subtypes | <0.001* | ||
| ŌĆāEmbo-LVO | 197 (96.1) | 38 (45.8) | |
| ŌĆāICAS-LVO | 8 (3.9) | 45 (54.2) | |
| 2b-3 Final mTICI achieved | 160 (78.0) | 67 (80.7) | 0.750 |
| mRS Ōēż2 at 90 days | 70 (34.1) | 35 (42.2) | 0.225 |
| sICH | 20 (9.8) | 4 (4.8) | 0.239 |
Values are presented as mean┬▒standard deviation or number (%).
TES, thrombus enhancement sign; NIHSS, National Institutes of Health Stroke Scale; ASPECTS, Alberta Stroke Program Early CT Score; MCA, middle cerebral artery; ICA, internal carotid artery; CBS, clot burden score; IVT, intravenous thrombolysis; embo-LVO, embolism-related large vessel occlusion; ICAS-LVO, intracranial atherosclerotic stenosis-related large vessel occlusion; mTICI, modified Thrombolysis in Cerebral Infarction; mRS, modified Rankin Scale; sICH, symptomatic intracerebral hemorrhage.
Table┬Ā2.
Comparison between patients with embo-LVO and ICAS-LVO
| Embo-LVO (n=235) | ICAS-LVO (n=53) | P | |
|---|---|---|---|
| Age (yr) | 71.3┬▒11.7 | 73.1┬▒11.2 | 0.331 |
| Male sex | 138 (58.7) | 26 (49.1) | 0.221 |
| Baseline NIHSS | 16.4┬▒5.3 | 14.8┬▒4.6 | 0.042* |
| Baseline ASPECTS | 9.6┬▒0.8 | 9.7┬▒0.4 | 0.373 |
| Risk factors | |||
| ŌĆāDiabetes mellitus | 61 (26.0) | 11 (20.8) | 0.486 |
| ŌĆāHypertension | 135 (57.4) | 36 (67.9) | 0.168 |
| ŌĆāAtrial fibrillation | 157 (66.8) | 9 (17.0) | <0.001* |
| ŌĆāCoronary artery disease | 72 (30.6) | 11 (20.8) | 0.180 |
| ŌĆāDyslipidemia | 43 (18.3) | 15 (28.3) | 0.128 |
| ŌĆāCurrent smoking | 41 (17.4) | 8 (15.1) | 0.840 |
| Occlusion sites | 0.073 | ||
| ŌĆāMCA | 161 (68.5) | 38 (71.7) | |
| ŌĆāICA | 74 (31.5) | 15 (28.3) | |
| TES | 197 (83.8) | 8 (15.1) | <0.001* |
| CBS | 6.5┬▒1.2 | 6.4┬▒1.2 | 0.268 |
| IVT | 94 (40.0) | 23 (43.4) | 0.646 |
| Onset to puncture (min) | 271.8┬▒150.1 | 259.7┬▒109.2 | 0.582 |
| Puncture to recanalization (min) | 55.3┬▒25.8 | 51.3┬▒24.6 | 0.300 |
| Procedure time (min) | 68.1┬▒25.6 | 115.4┬▒17.3 | <0.001* |
| 2b-3 Final mTICI achieved | 189 (80.4) | 38 (71.7) | 0.192 |
| mRS Ōēż2 at 90 days | 88 (37.4) | 17 (32.1) | 0.529 |
| sICH | 22 (9.4) | 2 (3.8) | 0.271 |
Values are presented as mean┬▒standard deviation or number (%).
embo-LVO, embolism-related large vessel occlusion; ICAS-LVO, intracranial atherosclerosis-related large vessel occlusion; NIHSS, National Institutes of Health Stroke Scale; ASPECTS, Alberta Stroke Program Early CT Score; MCA, middle cerebral artery; ICA, internal carotid artery; TES, thrombus enhancement sign; CBS, clot burden score; IVT, intravenous thrombolysis; mTICI, modified Thrombolysis in Cerebral Infarction; mRS, modified Rankin Scale; sICH, symptomatic intracerebral hemorrhage.
Table┬Ā3.
Factors associated with embo-LVO
Table┬Ā4.
Receiver operating characteristic curve analysis of embo-LVO patients
References
1. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723-1731.


2. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018;378:11-21.

3. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35-41.


4. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019;50:e344-e418.


5. Gutierrez J, Turan TN, Hoh BL, Chimowitz MI. Intracranial atherosclerotic stenosis: risk factors, diagnosis, and treatment. Lancet Neurol 2022;21:355-368.


6. Holmstedt CA, Turan TN, Chimowitz MI. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment. Lancet Neurol 2013;12:1106-1114.



7. Tsang ACO, Orru E, Klostranec JM, Yang IH, Lau KK, Tsang FCP, et al. Thrombectomy outcomes of intracranial atherosclerosis-related occlusions. Stroke 2019;50:1460-1466.


8. Jia B, Feng L, Liebeskind DS, Huo X, Gao F, Ma N, et al. Mechanical thrombectomy and rescue therapy for intracranial large artery occlusion with underlying atherosclerosis. J Neurointerv Surg 2018;10:746-750.


9. Vilela P, Rowley HA. Brain ischemia: CT and MRI techniques in acute ischemic stroke. Eur J Radiol 2017;96:162-172.


10. Kufner A, Erdur H, Endres M, Nolte CH, Scheel M, Schlemm L. Association between thrombus perviousness assessed on computed tomography and stroke cause. Stroke 2020;51:3613-3622.


11. Berndt M, Friedrich B, Maegerlein C, Moench S, Hedderich D, Lehm M, et al. Thrombus permeability in admission computed tomographic imaging indicates stroke pathogenesis based on thrombus histology. Stroke 2018;49:2674-2682.


12. Wei L, Zhu Y, Deng J, Li Y, Li M, Lu H, et al. Visualization of thrombus enhancement on thin-slab maximum intensity projection of CT angiography: an imaging sign for predicting stroke source and thrombus compositions. Radiology 2021;298:374-381.


13. He G, Deng J, Lu H, Wei L, Zhao Y, Zhu Y, et al. Thrombus enhancement sign on CT angiography is associated with the first pass effect of stent retrievers. J Neurointerv Surg 2023;15:146-152.


14. Humphries W, Hoit D, Doss VT, Elijovich L, Frei D, Loy D, et al. Distal aspiration with retrievable stent assisted thrombectomy for the treatment of acute ischemic stroke. J Neurointerv Surg 2015;7:90-94.


15. Puetz V, Dzialowski I, Hill MD, Subramaniam S, Sylaja PN, Krol A, et al. Intracranial thrombus extent predicts clinical outcome, final infarct size and hemorrhagic transformation in ischemic stroke: the clot burden score. Int J Stroke 2008;3:230-236.



16. Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke 2013;44:2650-2663.



17. Lee JS, Hong JM, Kim JS. Diagnostic and therapeutic strategies for acute intracranial atherosclerosis-related occlusions. J Stroke 2017;19:143-151.




18. Tomasello A, Rib├▓ M, Gramegna LL, Melendez F, Rosati S, Moreu M, et al. Procedural approaches and angiographic signs predicting first-pass recanalization in patients treated with mechanical thrombectomy for acute ischaemic stroke. Interv Neuroradiol 2019;25:491-496.




19. Lee JS, Hong JM, Lee KS, Suh HI, Choi JW, Kim SY. Primary stent retrieval for acute intracranial large artery occlusion due to atherosclerotic disease. J Stroke 2016;18:96-101.




20. Yoo J, Lee SJ, Hong JH, Kim YW, Hong JM, Kim CH, et al. Immediate effects of first-line thrombectomy devices for intracranial atherosclerosis-related occlusion: stent retriever versus contact aspiration. BMC Neurol 2020;20:283.




21. Suh HI, Hong JM, Lee KS, Han M, Choi JW, Kim JS, et al. Imaging predictors for atherosclerosis-related intracranial large artery occlusions in acute anterior circulation stroke. J Stroke 2016;18:352-354.




22. Kim YW, Hong JM, Park DG, Choi JW, Kang DH, Kim YS, et al. Effect of intracranial atherosclerotic disease on endovascular treatment for patients with acute vertebrobasilar occlusion. AJNR Am J Neuroradiol 2016;37:2072-2078.



23. Cho KH, Kim JS, Kwon SU, Cho AH, Kang DW. Significance of susceptibility vessel sign on T2*-weighted gradient echo imaging for identification of stroke subtypes. Stroke 2005;36:2379-2383.


24. Liebeskind DS, Sanossian N, Yong WH, Starkman S, Tsang MP, Moya AL, et al. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke 2011;42:1237-1243.



25. Kim SK, Yoon W, Heo TW, Park MS, Kang HK. Negative susceptibility vessel sign and underlying intracranial atherosclerotic stenosis in acute middle cerebral artery occlusion. AJNR Am J Neuroradiol 2015;36:1266-1271.



26. Baik SH, Kim JW, Kim BM, Kim DJ. Significance of angiographic clot meniscus sign in mechanical thrombectomy of basilar artery stroke. J Neurointerv Surg 2020;12:477-482.


27. Baek JH, Kim BM, Yoo J, Nam HS, Kim YD, Kim DJ, et al. Predictive value of computed tomography angiography-determined occlusion type in stent retriever thrombectomy. Stroke 2017;48:2746-2752.


28. Chen WH, Yi TY, Zhan AL, Wu YM, Lu YY, Li YM, et al. Stent-unsheathed effect predicts acute distal middle cerebral artery atherosclerotic disease-related occlusion. J Neurol Sci 2020;416:116957.


29. Kim JW, Lee BH. Usefulness of stent strut deformity during thrombectomy for predicting the stroke etiology in acute large artery occlusion. Clin Neurol Neurosurg 2020;198:106130.


30. Yi TY, Chen WH, Wu YM, Zhang MF, Zhan AL, Chen YH, et al. Microcatheter ŌĆ£first-pass effectŌĆØ predicts acute intracranial artery atherosclerotic disease-related occlusion. Neurosurgery 2019;84:1296-1305.


31. Al Kasab S, Almallouhi E, Spiotta AM. Rescue endovascular treatment for emergent large vessel occlusion with underlying intracranial atherosclerosis: current state and future directions. Front Neurol 2021;12:734971.



32. Menon BK, Al-Ajlan FS, Najm M, Puig J, Castellanos M, Dowlatshahi D, et al. Association of clinical, imaging, and thrombus characteristics with recanalization of visible intracranial occlusion in patients with acute ischemic stroke. JAMA 2018;320:1017-1026.



33. Mishra SM, Dykeman J, Sajobi TT, Trivedi A, Almekhlafi M, Sohn SI, et al. Early reperfusion rates with IV tPA are determined by CTA clot characteristics. AJNR Am J Neuroradiol 2014;35:2265-2272.



34. Lee JS, Hong JM, Lee KS, Suh HI, Demchuk AM, Hwang YH, et al. Endovascular therapy of cerebral arterial occlusions: intracranial atherosclerosis versus embolism. J Stroke Cerebrovasc Dis 2015;24:2074-2080.










