Occurrence of Ischemic Stroke in Patients With Atrial Fibrillation Receiving Non-Vitamin K Oral Anticoagulants: Causes and Prevention Strategies
Article information
Abstract
Atrial fibrillation (AF) is a leading cause of cardioembolic stroke, which is often fatal or disabling. Prevention of stroke is crucial in AF management, and anticoagulation with non-vitamin K oral anticoagulants (NOACs) is the mainstay of AF management for stroke prevention. Because NOAC prescriptions have been surging worldwide, the development of acute ischemic stroke in patients with AF who receive NOAC treatment is an increasingly important issue in clinical practice. Moreover, these patients show a high risk of recurrence, with more than a 50% higher risk, than do patients with AF and no prior anticoagulation therapy. Careful evaluation is mandatory to determine possible causes of ischemic stroke during NOAC therapy. Differentiation of AF-unrelated stroke and demonstration of combined cardiac disease/systemic coagulopathy are important in these patients and may provide improved results in their treatment. In addition, ensuring appropriate dosing and good adherence to NOAC treatment is important. Cardioembolism, despite sufficient anticoagulation and no other causes, is the most common and challenging complication because switching to anticoagulants or adding antiplatelets to the treatment regimen does not reduce the risk of recurrent stroke, and there are no guidelines for this specific situation. This review article aimed to present the most updated data on the prevalence, causes, and secondary prevention strategies, specifically focusing on non-pharmacological approaches, together with relevant cases of AF in patients who developed ischemic stroke on NOAC therapy.
Introduction
Atrial fibrillation (AF) is a leading cause of cardioembolic stroke, which is often fatal or disabling. Prevention of stroke is crucial in AF management, and oral anticoagulants (OAC) are mainly used for stroke prevention in patients with AF. Four pivotal randomized controlled trials (RCTs) of non-vitamin K OACs (NOACs)—apixaban, dabigatran, edoxaban, and rivaroxaban—showed non-inferiority to vitamin K antagonists (VKA) in the prevention of stroke/systemic embolism (SE) in patients with AF [1-4]. The prescription of NOACs has increased over time. The increase in the use of NOACs for stroke prevention in patients with AF has largely been driven by no need for monitoring of the international normalized ratio (INR) and the reduced risk of intracranial/major bleeding. The occurrence of ischemic stroke in patients with AF who are receiving NOACs presents clinicians with a great challenge in selecting the optimal therapeutic strategy. This is because NOACs are considered the gold standard in the prevention of ischemic stroke in patients with non-valvular AF (NVAF); however, there are no guidelines for this specific situation [5,6].
Recently, data on the characteristics of patients with AF who develop ischemic stroke despite NOAC therapy have been accumulating. In addition, significant advances in non-pharmacological approaches to prevent stroke in patients with AF who are ineligible for NOAC treatment have taken place. This review aimed to present the most updated data on the prevalence, causes, and secondary prevention strategies, specifically focusing on nonpharmacological approaches, in patients with AF who developed ischemic stroke while taking NOACs.
Systematic review
For this review, we searched PubMed and ClinicalTrials.gov for relevant references of studies published in English up to July 2022 using the search terms atrial fibrillation, stroke, dabigatran, rivaroxaban, apixaban, edoxaban, NOAC failure, DOAC failure, and left atrial appendage. Additionally, we identified references in relevant articles and reviews. The final reference list was generated on the basis of originality and relevance to this topic.
Increasing prevalence of patients with AF with NOAC failure
Recent studies have shown that 20%–36% of ischemic strokes in patients with AF occur while taking VKAs or NOACs: 20.1% in real-world multicenter data of Korea–Japan [7], 36.0% in a retrospective multicenter study [8], and 22.5% in a prospective multicenter study [9]. Thus, this is a common scenario in routine stroke care.
Auer et al. [10] showed that while the percentage of acute ischemic stroke with preceding VKA usage is steadily decreasing, the number of patients with preceding NOAC therapy is on the rise. The proportion of patients who develop ischemic stroke with NOAC therapy is expected to increase over the years for the following reasons. First, a recent meta-analysis showed that the worldwide proportion of OAC users (either VKAs or NOACs) among patients with AF almost doubled from 42% in 2010 to 78% in 2018, and the proportion of newly diagnosed patients with AF starting OACs increased from 43% to 75% [11]. However, since one-quarter of OAC-eligible patients with AF did not receive anticoagulants in 2018, the proportion of NOAC use in patients with AF will increase with time. Second, among patients with AF taking anticoagulants, the proportion of NOAC use will continuously increase. The proportion of patients with AF using NOACs increased globally from 0.00 (95% confidence interval [CI] 0.00–0.00) in 2010 to 0.45 (95% CI 0.45–0.46) in 2018, whereas the proportion of VKA users slightly decreased from 0.42 (95% CI 0.22–0.65) in 2010 to 0.32 (95% CI 0.32–0.32) in 2018 [11]. Some intercountry differences were observed. The prevalence of NOAC use is generally lower in Asia than that in North America or Europe. Yu et al. [12] evaluated the pharmacy prescription data of five major cities in China during a similar period (2012–2017) and showed that the use of NOACs was increasing rapidly; however, the percentage of patients receiving VKAs remained higher (from 98% in 2012 to 72% in 2017 among patients receiving OACs) owing to the cost factor. Lastly, the proportion of the elderly population is increasing, especially in Asia and developing countries, and the number of AF cases is predicted to increase in Asia [13].
A higher risk of recurrence of ischemic stroke
Seiffge et al. [9] conducted a pooled analysis of individual patient data from seven prospective cohort studies of 5,314 patients. They found that patients with AF who had ischemic stroke despite prior OAC therapy (VKA or NOAC) were at a higher risk of recurrent ischemic stroke despite a CHA2DS2-VASc score similar to those without prior OAC. Prior OAC use was associated with an increased risk of acute ischemic stroke (hazard ratio [HR] 1.6, 95% CI 1.2–2.3). The annual rate of recurrent ischemic stroke was twice as high in patients who had index stroke despite taking OACs (8.9%) than in those who were not on OAC therapy at the time of index stroke (3.9%). In the Initiation of Anticoagulation after Cardioembolic Stroke (IAC) study, which is a multicenter retrospective study, a higher risk of 90-day recurrent ischemic stroke was observed after adjusting for potential confounders among patients prior to OAC use than among those with no OAC use prior to index stroke (HR 1.50, 95% CI 0.99–2.28) [8]. In the RENO-EXTEND (Causes and Risk Factors of Cerebral Ischemic Events in Patients With Nonvalvular AF Treated with NOACs for Stroke Prevention-Extend) study, which is a prospective multicenter observational study, patients with stroke despite being on NOAC therapy were at a high risk of recurrent ischemic stroke and bleeding [14]. In patients with stroke while on treatment with NOACs, the annual rate of thromboembolic and bleeding events was higher (13.4%) in this study than in previous pivotal trials of NOACs (2.83% in ENGAGE AF-TIMI 48 [Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis in Myocardial Infarction 48], 2.07%–2.32% in RELY [Randomized Evaluation of Long-Term Anticoagulant Therapy Trial], 1.01% in ARISTOTLE [Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation Trial], and 2.79% in ROCKET AF [Rivaroxaban Once Daily Oral Direct Factor Xa Inhibitor Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation]). A study by CRCS-K (Clinical Research Collaboration for Stroke in Korea) and SAMURAI (Stroke Acute Management With Urgent Risk-Factor Assessment and Improvement) study investigators comprised pooled individual patient data on AF-associated acute ischemic stroke/transient ischemic attack (TIA) from 5,645 patients [7]. The risk of recurrent ischemic stroke was higher in patients with AF-associated stroke with prior OAC than in those without prior OAC (the cumulative incidence was 5.3% vs. 2.9%, HR 1.50, 95% CI 1.02–2.20). Subgroup analysis showed an increased risk of recurrent stroke in both prior VKA and NOAC users.
In all aforementioned studies, no significant difference was observed in the risk of hemorrhagic stroke between patients with prior OAC therapy and those with no prior anticoagulation therapy before index stroke.
Causes and risk factors
AF-unrelated stroke
Although carotid intervention is the mainstay treatment for symptomatic carotid stenosis, not all recurrent stroke in patients with carotid stenosis are due to a carotid origin. Likewise, AF is not always an etiology of stroke in patients with AF and ischemic stroke. A sub-analysis of a prospective, open-label, multicenter, post-marketing surveillance study in South Korea of 651 patients with stroke and AF treated with apixaban showed that patients with cerebral atherosclerotic lesions had a higher rate of major events than did those without (4.6% vs. 1.7%, P=0.0357) [15]. In this study, 30.8% of recurrent strokes were potentially caused by large artery diseases, and over half of the patients who experienced recurrent stroke caused by cardiogenic embolism had atherosclerosis on magnetic resonance angiography. We have shown that approximately one in six of patients with ischemic stroke and AF are unrelated to AF (carotid stenosis on the relevant site or small vessel disease) and have distinct characteristics compared with AF-related stroke [16]. Patients with AF-unrelated stroke often experienced recurrence during appropriate anticoagulation therapy. The proportion of AF-unrelated stroke may be higher in patients with acute stroke during NOAC therapy. In the RENo (Causes and Risk Factors of Cerebral Ischemic Events in Patients With Nonvalvular AF Treated With NOACs for Stroke Prevention) study, about one-third of patients with AF who had an acute ischemic stroke during NOAC treatment experienced non-cardioembolic stroke as a recurrence [17]. In the IAC study, 16.4% had atherosclerotic stenosis >50% [8]. Anticoagulants alone may not be sufficient to prevent stroke in patients with unrelated AF. Thus, the differentiation between AF-related and unrelated stroke is important for optimizing treatment against AF-unrelated stroke mechanisms, such as the use of antiplatelets or statins.
We compared morphometric and volumetric parameters of the left atrial appendage (LAA) on multidetector cardiac computed tomography (CT) between patients with AF-related and unrelated strokes [18]. Among patients with AF plus acute ischemic stroke, larger LAA orifice diameter and LAA volume were independently associated with AF-related stroke, suggesting the role of cardiac imaging of LAA for stratifying non-cardioembolic risk in patients with AF (Figure 1).
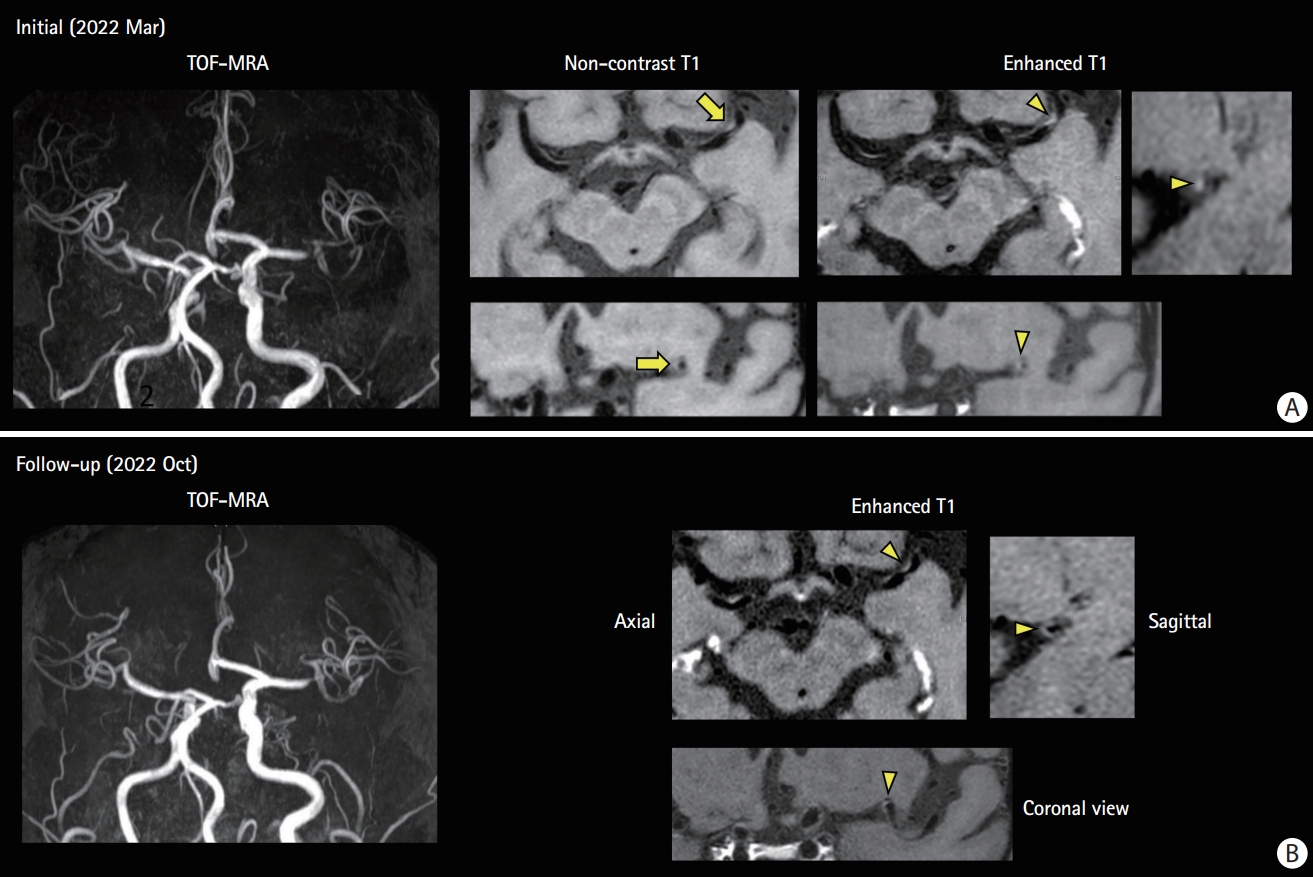
Competing mechanism: high-resonance magnetic resonance imaging (MRI) findings of a 69-year-old man with atrial fibrillation on anticoagulated with edoxaban. (A) He experienced transient right hemiparesis. He was taking edoxaban with good adherence. Magnetic resonance (MR) angiogram shows focal stenosis on the distal M1 segment. No acute infarct is observed on diffusion-weighted imaging and MR perfusion showed perfusion delay on the left middle cerebral artery hemisphere. High resonance MRI shows intraplaque hemorrhage on T1-weighted images (arrow) and eccentric wall thickening and enhancement on contrast-enhanced T1-weighted images (arrowhead) suggesting unstable intracranial plaque. The echocardiogram showed no cardiac thrombi and left atrial enlargement was not prominent. Clopidogrel and a high-dose statin were added to edoxaban. (B) A follow-up high-resolution MRI at 6 months of treatment. Plaque enhancement disappears (arrowhead). TOF-MRA, time-of-flight magnetic resonance angiography.
Poor compliance or underdosing
Non-adherence to prescribed NOACs could be a cause of stroke while taking NOACs. A large US commercial insurance database of 64,661 patients with AF showed that adherence to OAC therapy is poor and modestly improved with NOACs [19]. In this study, 47.5% of patients taking NOAC had proportion of days covered (PDC) ≥80%, and poor adherence to therapy was associated with an increased risk of stroke, especially in patients with CHA2DS2-VASc score ≥2. Similarly, a population-based cohort study using the French national healthcare data system showed that adherence to NOAC treatment (defined as PDC ≥80%) reached 66.6% of patients during the 2-year follow-up period [20]. Of 76,795 patients with AF newly treated with NOACs, non-adherence was associated with an increased risk of stroke and death. Coagulation tests or blood levels of NOACs can provide information on patient compliance, such as prolonged activated partial thromboplastin time and thrombin time in response to the level of dabigatran and prolonged prothrombin time and anti-Xa levels in response to the level of factor Xa inhibitors [21]. In patients with non-adherence without intolerance, once-daily dosing of NOAC with rivaroxaban or edoxaban could be an option to improve adherence to NOACs. However, a relatively high failure rate of rivaroxaban compared with other NOACs was reported in a review of 79 patients with NOAC failure [22]. The high rate of failure in rivaroxaban use may be related to inadequate anticoagulation due to its relatively fast elimination rate and once-a-day dosing.
The use of lower doses than those recommended in drug data sheets is not uncommon, especially in Asian populations [13]. A US nationwide AF registry showed that reduced NOAC dose was prescribed to 16% of patients with AF [23]. Inappropriate prescription of reduced doses of NOACs has been associated with increased risk of stroke/SE, hospitalization, and deaths without appreciable reductions in major bleeding in both Asians and non-Asians [23,24]. In the RENo study, the main risk factor associated with an increased risk of ischemic cerebrovascular events was the administration of a low dose of NOACs [17]. Dose selection criteria for NOACs are different among NOACs: age ≥80, use of verapamil, and increased bleeding risk for dabigatran; creatinine clearance (CrCl) 15–49 mL/min for rivaroxaban; age ≥80, body weight ≤60 kg, or serum creatinine ≥1.5 mg/dL for apixaban; and CrCl 15–50 mL/min, body weight ≤60 kg, and use of dronedarone, cyclosporines, erythromycin, or ketoconazole for edoxaban [5]. Therefore, although no head-to-head comparison study has been conducted, switching from a lower dose NOAC to a higher dose NOAC as per the dose selection criteria could be considered in patients with NOAC failure.
Drug interactions could be another pharmacokinetic cause of NOAC failure [25]. NOACs are substrates for p-glycoprotein in the gut, and the metabolism of rivaroxaban and apixaban is partially dependent on the liver enzyme cytochrome P450 3A4 (CYP3A4). Drug interactions may occur when p-glycoprotein and CYP3A4 inducers are used concomitantly with NOACs. For example, in Asian patients with AF undergoing NOAC therapy, concurrent use of enzyme-inducing antiepileptic drugs was associated with an increased risk of recurrent ischemic stroke or TIA [26]. In these cases, either adjustment of the dose of NOACs or switching of concomitant drugs to drugs with no known drug interaction could be considered.
Other combined illness
Combined cardiac or systemic illness should be suspected in patients with AF after treatment failure with NOAC. Valvular AF is defined as AF related to rheumatic valvular disease or prosthetic heart valve. The pivotal NOAC trials included a substantial number of patients with valvular heart diseases. The sub-studies of pivotal trials compared the effects of NOACs and VKA in patients with AF and valvular heart disease and supported the use of NOACs in patients with AF and valvular heart disease, excluding only those with prosthetic valve disease or hemodynamically significant mitral stenosis [27,28]. According to the guidelines on the use of NOAC in patients with AF, NOAC therapy is eligible for mild-to-moderate valvular disease, bioprosthetic valves, and severe atrial stenosis [25]. However, a recent RCT showed that among patients with rheumatic heart disease-associated AF, VKA led to a lower rate of cardiovascular events and death than rivaroxaban therapy did [29]. Therefore, in patients with AF who develop ischemic stroke on NOAC treatment, echocardiographic evaluation is needed to demonstrate the presence/severity of valvular heart disease.
Coagulopathy may coexist in patients with AF. Among the causes of coagulopathy, malignancy and antiphospholipid antibody syndrome (APS) have been reported to be associated with NOAC failure [22,26]. Some retrospective studies showed that compared with VKA, NOAC was associated with a lower risk of stroke/SE and major bleeding in patients with AF and cancer [30,31]. However, these are the results of safety and efficacy of NOAC in preventing AF-related stroke/SE in patients with cancer, and they could not directly be transferred to the role of NOACs in preventing cancer-associated thromboembolism. Lin et al. [26] showed that patients with AF presenting with underlying malignancy had an increased risk of treatment failure with NOAC in secondary stroke prevention. APS is an acquired thrombophilic disorder in which both arterial and venous thromboses are associated with the presence of persistent antiphospholipid antibodies. Anticoagulation with VKA remains the standard treatment for the secondary prevention of thrombosis in patients with APS. In an RCT, compared with the use of VKA, the use of rivaroxaban was associated with an increased risk of stroke in patients with APS (relative risk 19.00, 95% CI 1.12–321.9) [32]. A recent meta-analysis showed that patients with thrombotic APS randomized to NOACs compared with VKAs had increased risk for arterial thrombosis (odds ratio [OR] 5.43, 95% CI 1.87–15.75), especially stroke [33].
Cardioembolism despite sufficient anticoagulation and no causes
This is the most common and challenging entity in the selection of optimal pharmacological and non-pharmacological strategies to prevent recurrent ischemic stroke. Polymeris et al. [34] analyzed 2,946 patients from a multinational prospective registry and showed that stroke despite anticoagulation has heterogeneous etiologies with cardioembolism despite sufficient anticoagulation being the most common (44%), followed by insufficient anticoagulation (32%) and competing mechanisms (24%). Although appropriate anticoagulation at the time of stroke onset was associated with better functional outcome and smaller infarct volume [35-37], the higher risk of ischemic stroke recurrence in patients with AF on NOAC failure may result in severe neurological disability (Figure 2).
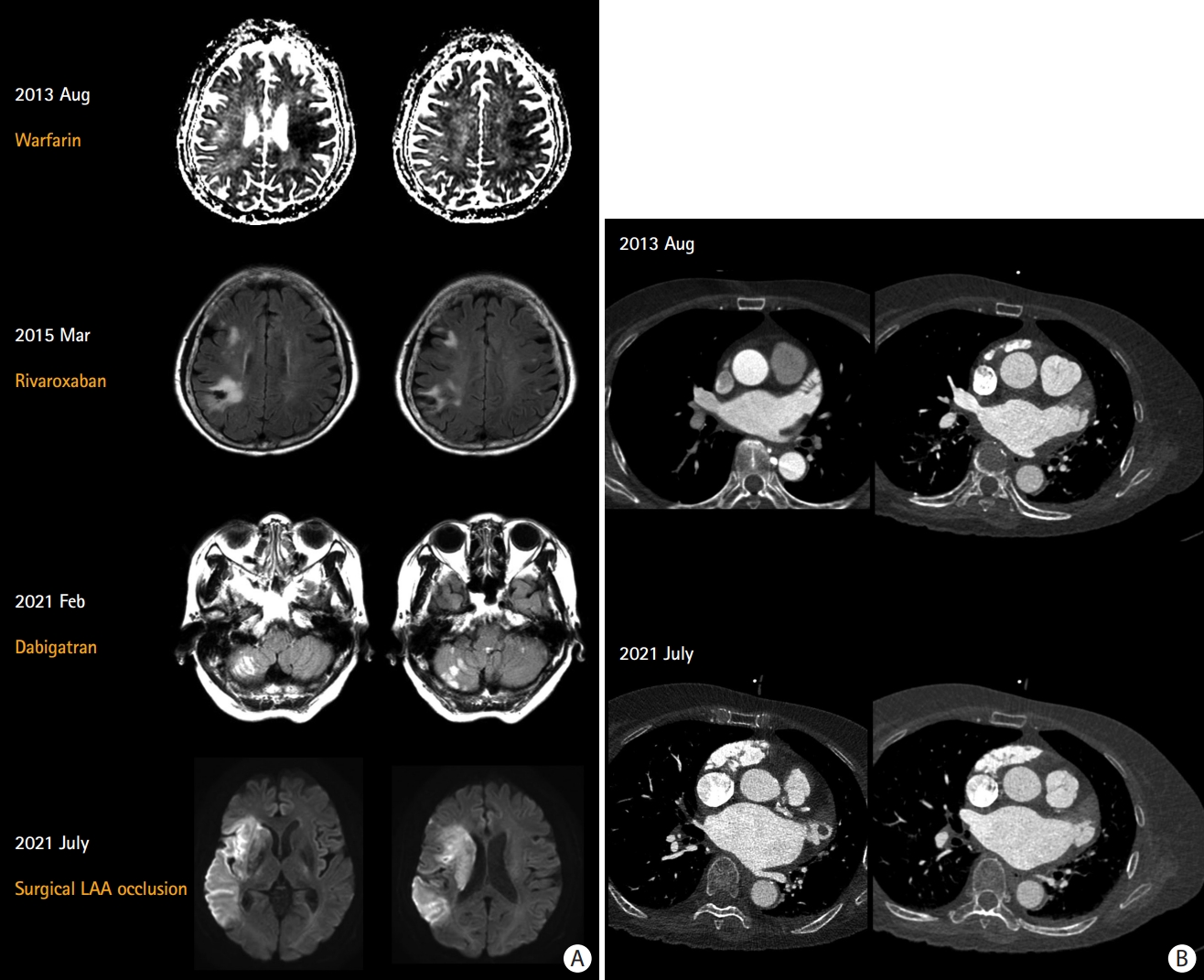
Recurrent cardioembolism despite sufficient anticoagulation: a 76-year-old woman experienced recurrent stroke despite sufficient anticoagulation. (A) Warfarin was switched to rivaroxaban owing to poor international normalized ratio control. She was a CYP2C9 *1/1* and VKORC1 TT carrier suggesting extensive metabolizer and high warfarin sensitivity. Dabigatran was started after recurrent stroke despite good adherence to rivaroxaban. Finally, surgical LAA exclusion and Maze operation were performed. (B) Serial multidetector cardiac computed tomography shows an enlargement of LA and LAA volume and the development of a 2 cm-sized LAA thrombus. Echocardiography also showed increased LA volume index from 44 mL/m2 to 57 mL/m2. LAA, left atrial appendage; LA, left atrium.
High CHA2DS2-VASc scores and the presence of individual components of the scoring system (e.g., heart failure and prior stroke) were associated with the occurrence of ischemic stroke in patients with AF on NOACs. The RENo study investigators compared the characteristics of 713 patients with AF who had a stroke during NOAC therapy with those of 700 patients with AF who did not have a stroke during NOAC therapy to investigate the pathogenesis and risk factors for a stroke occurring during NOAC therapy for stroke prevention [17]. They showed that off-label low doses, atrial enlargement, hyperlipidemia, and a high CHA2DS2-VASc score were associated with an increased risk of cerebrovascular events during NOAC therapy. In the PREFER in AF (PREvention oF thromboembolic events–European Registry in Atrial Fibrillation) study, which is a prospective, multicenter observational study of 461 centers in seven European countries, several modifiable factors, including prior stroke/TIA, concomitant therapy with antiplatelet/non-steroidal anti-inflammatory drugs, heart failure, and old age, were associated with both thromboembolic and major bleeding events in patients receiving an NOAC [38]. The CRCS-K and SAMURAI study investigators showed that congestive heart failure was more frequent in patients with prior anticoagulation than in those without prior OAC (14.8% vs. 6.6%, P<0.01), suggesting the high-risk nature of the prior OAC group [7].
In addition to the CHA2DS2-VASc score, impaired renal function was associated with NOAC failure. Patients with AF often show decreased estimated glomerular filtration rates (eGFR), and chronic kidney disease (CKD) could be a predictor of LAA thrombus in patients taking NOACs. Budnik et al. [39] evaluated transesophageal echocardiography (TEE) in patients with AF who were taking NOACs. They demonstrated that despite NOAC use, patients with concomitant AF and CKD had a high risk for LAA thrombus formation. In this study, among patients prescribed reduced doses of NOAC, those with decreased eGFR were more often observed with an LAA thrombus (10% vs. 2.5%). Michalska et al. [40] studied TEE in patients with AF treated with NOACs. They revealed that the CHA2DS2-VASc-RAF (R is renal dysfunction, and AF is atrial fibrillation type) score, which incorporated an additional assessment of the form of AF (paroxysmal vs. persistent vs. permanent) and impaired renal function (CrCl and eGFR), had a better predictive ability to distinguish between patients with and without an LAA thrombus than did the CHA2DS2-VASc score. Further studies are needed, including blood biomarker levels, to predict cardioembolism in patients with AF taking NOACs.
Left atrial (LA) and LAA features are associated with the occurrence of ischemic stroke/TIA and the presence of an LA/LAA thrombus in patients taking NOACs. Yaghi and IAC study investigators showed that severe LA enlargement was more prevalent in patients with prior anticoagulant use than in those with no anticoagulant use prior to index stroke (43.9% vs. 34.9%, P=0.002) [8]. Some TEE studies reported the presence of a thrombus in LA or LAA despite anticoagulation. Shah et al. [41] compared clinical and TEE risk factors between patients with AF without stroke having an LAA thrombus despite NOAC prescription and those without an LAA thrombus. LA dilation, greater CHA2DS2-VASc score, absence of severe mitral regurgitation, and lower left ventricular ejection fraction were associated with an LAA thrombus despite NOAC therapy. Durmaz et al. [42] showed that in patients with AF and OAC therapy, CHA2DS2-VASc score, LA volume, LA flow velocity, and LV ejection fraction were associated with an LA thrombus, and patients with an LA thrombus experienced more ischemic strokes than did patients without an LA thrombus. Slow LAA emptying velocities were also associated with the presence of an LAA thrombus despite NOAC therapy [41,43]. There has been recent interest in the association between both LAA morphology and thrombi. A TEE study of 564 patients with AF without stroke showed that the complex LAA morphology, characterized by an increased number of LAA lobes, is associated with the presence of an LAA thrombus independent of clinical risk and blood stasis [44]. Most patients with an LAA thrombus had ≥3 LAA lobes.
Therapeutic strategies
Medical management to prevent cardioembolism in NOAC failure
Fastner et al. [45] conducted a survey of German stroke units about strategies for prophylaxis after an acute ischemic stroke in a patient with AF despite NOAC treatment. They showed that practitioners are more likely to switch from VKA to NOAC than vice versa and are very likely to switch between different NOACs depending on individual patient factors (e.g., coagulation tests).
Several case series have shown successful resolution of LAA thrombi with dabigatran, a factor IIa inhibitor that had been refractory to rivaroxaban, a factor Xa inhibitor [46,47]. In these studies, rivaroxaban did not diminish atrial thrombi, which could be eliminated by switching to dabigatran on serial echocardiographic studies. The reason for the disparate responses to NOACs remains unclear. Variability in the sensitivity of the target molecules to specific NOACs could be explained by the fact that factor IIa inhibitors can bind to and inhibit the activity of both soluble and fibrin-bound thrombin.
Several observational studies have investigated whether changes in OACs are associated with a reduced risk of stroke recurrence in patients receiving anticoagulation therapy before the stroke. Seiffge et al. [9] showed that changes in OACs (either VKA to NOAC, NOAC to VAK, or change in the type of NOACs) were not associated with a decreased risk of ischemic stroke. The IAC study investigators also showed that switching the anticoagulation class was not associated with a reduced risk of a recurrent ischemic event (HR 0.41, 95% CI 0.12–1.33) [8]. In the RENO-EXTEND study, the rates of ischemic and bleeding events were not different in patients irrespective of changing the original NOAC treatment (OR 1.2, 95% CI 0.8–1.7) [14]. Polymeris et al. [34] showed that while NOACs were associated with better outcomes than VKAs, adding antiplatelets to the treatment regimen was linked to worse outcomes. Switching between different NOAC types was not associated with better outcomes. Similarly, Tokunaga and the SAMURAI study investigators evaluated 1,189 patients with ischemic stroke/TIA and AF and showed that compared with no prior OACs, prior VKA administration with an INR ≥2 on admission was associated with a higher risk of ischemic stroke within 2 years (HR 2.94, 95% CI 1.20–6.15) [48]. The results of these studies suggest that better prevention strategies (either pharmacological or non-pharmacological) are needed for this high-risk patient group [9].
In summary, to our knowledge, no evidence indicates that the addition of antiplatelet agents to OACs or supratherapeutic INR improves outcomes in secondary stroke prevention. In addition, no study has prospectively evaluated the efficacy of the change in OAC therapy (either from NOAC to VKA or from one NOAC to another NOAC), and further RCT studies are needed for patients with cardioembolic stroke, despite sufficient anticoagulation.
Genetic variability could cause susceptibility to recurrent stroke in patients with AF and OAC use. CYP2C9 and VKORCI genes play a role in the response to VKAs, especially in Asian patients [49], but no such variability in the response to NOACs is known. Measurement of plasma drug levels could be a simple solution in cases where there is suspicion of under or overexposure to a certain drug. However, NOAC concentrations are not suggested by guidelines because there are no data on the possible benefits, in terms of efficacy and safety, of a possible NOAC dose adjustment based on plasma concentration [50].
Percutaneous LAA occlusion: past, present, and future perspectives
More than 90% of atrial thrombi in patients with non-rheumatic AF originate in the LAA [51,52]. In this context, catheter-based left atrial appendage occlusion (LAAO) is considered an alternative strategy for the prevention of thromboembolic complications in patients with AF and contraindications for long-term OAC [5].
In 2001, Sievert et al. [53] performed the first percutaneous LAAO using the PLAATO device (Appriva Medical, Sunnyvale, CA, USA). Since then, many LAAO devices have been developed, and techniques for implantation have also been improved. Currently, catheter-based LAAO devices are classified into three types based on three principles: plug, pacifier, and ligation [54]. The WATCHMAN (Boston Scientific, Marlborough, MA, USA) is the most commonly used percutaneous LAAO plug device that obstructs the neck of the LAA using a trans-septal approach. WATCHMAN is the only device that has demonstrated non-inferiority of antithromboembolic efficacy and safety compared with VKA therapy in patients with NVAF in RCTs (PROTECT AF [WATCHMAN Left Atrial Appendage System for Embolic PROTECTion in Patients With Atrial Fibrillation] and PREVAIL [Prospective Randomized Evaluation of the WATCHMAN LAA Closure Device In Patients With Atrial Fibrillation Versus Long Term Warfarin Therapy]) [55,56]. Two prospective registry studies also showed that the WATCHMAN device could lower the stroke risk in patients with contraindications to OAC [57,58]. Recently, the next-generation WATCHMAN FLX device designed to improve the safety of the procedure was evaluated in a single-arm prospective registry, and it is Food and Drug Administration-approved [59]. The Amplatzer devices (Cardiac Plug and Amulet, both Abbott Vascular, Santa Clara, CA, USA) are the second most commonly used LAAO devices that have a lobe and an additional disc to seal the LAA ostium from the LA side. The Amulet occluder showed non-inferiority for safety and effectiveness compared with the WATCHMAN device in an RCT [60]. Furthermore, LAAO devices including the WATCHMAN and Amulet showed non-inferior efficacy to NOACs in patients with AF (PRAGUE-17 [Left Atrial Appendage Closure vs. Novel Anticoagulation Agents in High-risk Patients With Atrial Fibrillation]) [61]. The LARIAT system (SentreHEART, Inc., Redwood City, CA, USA) uses both the endocardial and epicardial approaches to snare and ligate the LAA under magnetic guidance. Recently, pulmonary vein isolation plus LAA ligation using the LARIAT device in patients with persistent AF showed comparable rhythm outcomes compared with pulmonary vein isolation alone [62]. The LAAO devices and sample cases are provided in Figure 3.
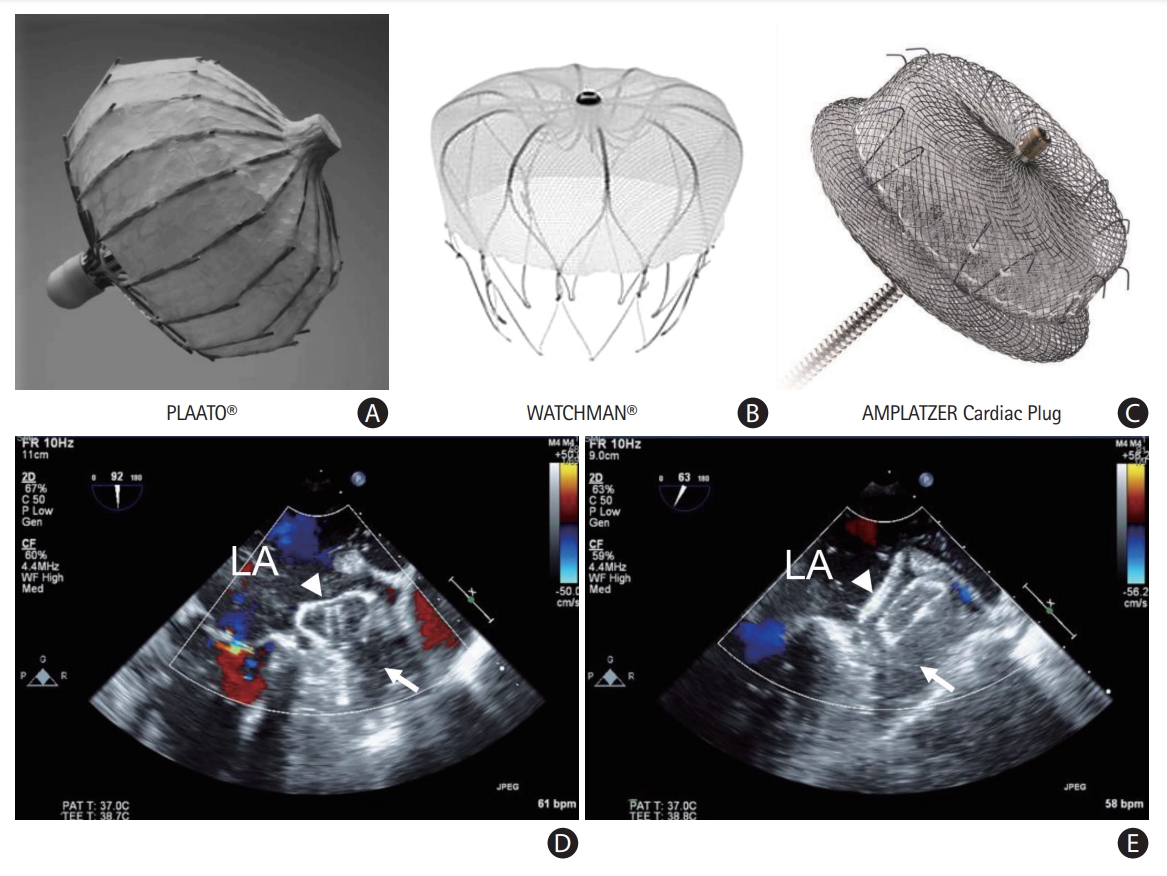
Percutaneous left atrial appendage (LAA) occluder devices: (A) PLAATO, (B) WATCHMAN, and (C) Amplatzer. Echocardiographic views of the devices: (D) WATCHMAN and (E) Amplatzer. LA, left atrium; white arrow, LAA; white triangle, LAA occluder device.
Considering the procedural aspects, pre- and post-procedural imaging between 1 and 6 months with either cardiac CT angiography or TEE are recommended to rule out pre-existing LAA thrombus and detect device-related thrombus (DRT) and peri-device leak, respectively [54]. In patients with contraindications for OAC or a high risk of bleeding, aspirin plus clopidogrel may be used for 1 to 6 months, followed by aspirin alone indefinitely [55,58]. Patients with DRT or significant peri-device leak over 5 mm should be considered to intensify anticoagulation therapy [54,55]. Despite advances in technology, catheter-based LAAO has several limitations. Patients with incomplete LAAO have a high thromboembolic risk. The incidence of DRT is not negligible. Fauchier et al. showed that the incidence of DRT after LAAO was 7.2% per year, and DRT was an independent predictor of stroke/TIA during follow-up, but it was lower after the use of newer devices (1.75% within 1 year of follow-up) [59,63].
The following issues need to be addressed by future randomized studies. First, effectiveness and safety of catheter-based LAAO compared with NOACs should be evaluated in patients with NOAC failure. Antithrombotic therapy after LAAO has never been evaluated in RCTs. Thus, current guidelines recommend that LAAO may be considered for stroke prevention in patients with AF who have contraindications for long-term OAC and high bleeding risk under chronic OAC (Class IIb) [5,54]. Based on the results of future RCTs, the role of LAAO in stroke prevention in patients with AF is expected to be expanded with more robust evidence. Few studies have investigated the role of percutaneous LAAO in patients with AF and recurrent stroke treated with adequate OACs. The role of percutaneous LAAO may be different between patients with AF having contraindications for OACs or high risk of bleeding and those with AF and recurrent stroke while on adequate OAC, because percutaneous LAAO may reduce the risk of hemorrhagic stroke but increase ischemic stroke/SE relative to VKAs [64]. Second, optimal antithrombotic therapy after the LAAO procedure should be investigated in patients with high thromboembolic risk, such as NOAC failure. Galloo et al. [65] conducted a multicenter retrospective cohort study and evaluated the efficacy, safety, and mortality of LAAO in patients with AF with recurrent stroke despite ongoing OAC, after the exclusion of other plausible causes. In this study, OAC was continued long-term after LAAO in most cases. They suggested that LAAO may be considered an adjunctive, but not an alternative, treatment to OAC with high feasibility and safety in OAC failure.
Surgical LAA occlusion: from anticoagulation ineligible patients to NOAC failure
Standalone or concomitant surgical LAAO during cardiac surgery has been performed for decades. To avoid the invasiveness of conventional cardiac surgery, minimally invasive LAAO, including totally thoracoscopic or da Vinci robotic cardiac surgery systems, has been performed. Currently, three methods are available to occlude the LAA: internal obliteration, epicardial resection with a stapler, and epicardial occlusion with clip devices (Figure 4).
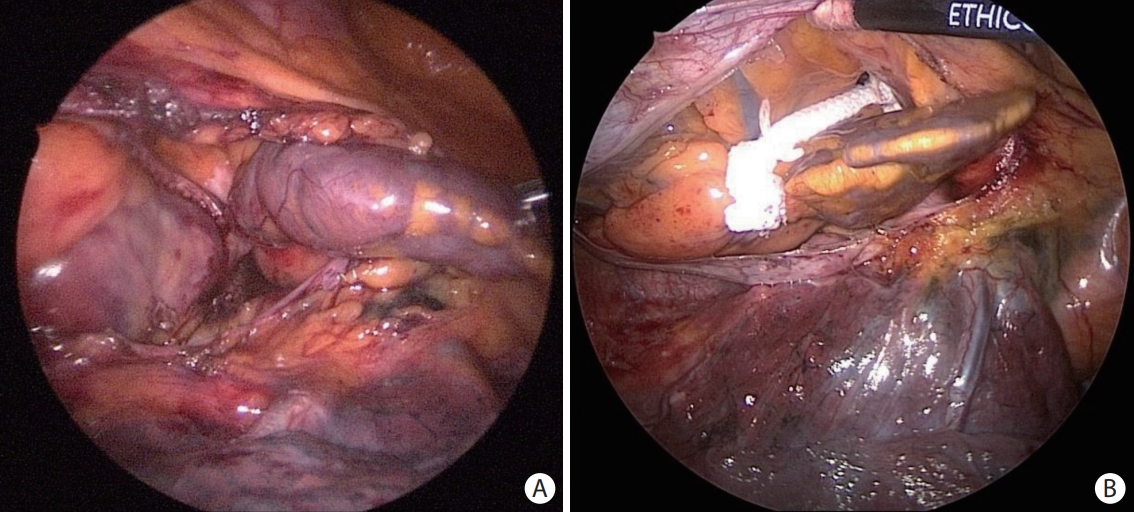
Epicardial left atrial appendage (LAA) exclusion: resection and occlusion. (A) Epicardial resection using a stapler. LAA is resected using a stapling device. A suture line at the base of LAA demonstrates no residual stump. (B) Epicardial occlusion with clip devices. LAA is occluded using a white-color clip device at the base of LAA. LAA is left unresected.
Endocardial suture obliteration of the LAA is an invasive procedure that requires the use of cardiopulmonary bypass, with an associated risk of bleeding and injury to the adjacent structures around the mitral annulus, such as the circumflex coronary artery. In addition, it is incomplete in 10%–30% of patients [66].
In a landmark study in 2008, different surgical techniques were used to evaluate surgical LAAO from 1993 to 2004 [67]. The LAA was amputated in 52 patients (38%) and was excluded in 85 patients (62%). Interestingly, 73% of patients who underwent excision had complete LAA occlusion, whereas a remnant LAA stump >1 cm was observed in 14 patients. The success rate of suture exclusion was only 23%.
Because of the low success rate of LAA exclusion using sutures, LAA resection using staplers or occlusion using a clip system is being adopted. The AtriClip device (AtriCure, Inc., Mason, OH, USA) is the first approved device for surgical LAA exclusion and has been deployed in more than 300,000 patients worldwide. This is a self-closing implantable clip made of two parallel titanium bars, connected with nitinol hinges and covered in a braided polyester lining. The clip may be repositioned if the initial placement is inadequate. After deployment, it produces a constant compression pressure, ensuring the complete exclusion of the LAA. This retrospective analysis assessed the long-term LAA efficacy of the AtriClip device applied via a totally thoracoscopic approach with chest CT [68]. Complete LAA closure (no exposed trabeculations and residual stump <1 cm) was observed in 61/65 participants (93.9%). No major complications were noted.
A recent meta-analysis of the AtriClip system found that complete closure was 97.8%, and there were no periprocedural device-related adverse events in 922 patients [69]. Stroke rates ranged from 0.2 to 1.5 per 100 patient-years in the follow-up, with stopped anticoagulation in 59% [69]. A long-term study showed that in patients who discontinued OAC during follow-up, the relative stroke risk was reduced by 87.5%, with an observed ischemic stroke rate of 0.5 per 100 patient-years, compared with the rate expected in a group of patients with similar CHA2DS2-VASc scores (4.0 per 100 patient-years) [70]. In all patients, the LAA was completely obliterated, and no device-related complications were detected throughout the follow-up period. Furthermore, CT imaging in selected patients (performed 5.1–8.1 years after device implantation) showed long-term durability with complete LAA occlusion and no signs of residual reperfusion or substantial LAA stumps. The most recent study of 460 patients evaluating the 10-year efficacy of totally thoracoscopic LAAO using AtriClip devices demonstrated that the annualized incidence rate of ischemic stroke was 0.78%/year, showing a 73% risk reduction compared with the CHA2DS2-VASc predicted rate without anticoagulation (Figure 5) [71].
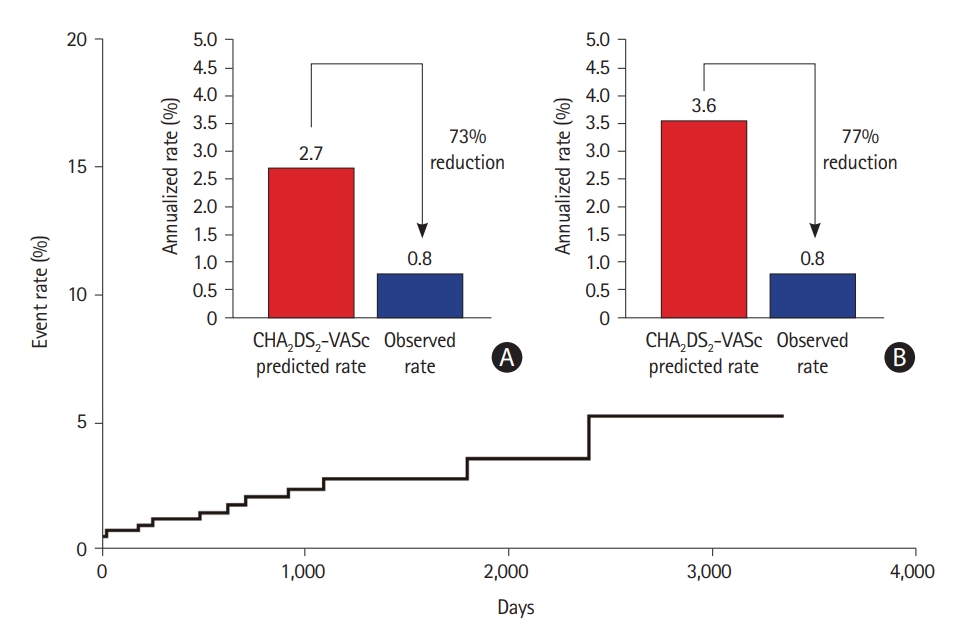
Relative reduction of ischemic stroke with surgical left atrial appendage occlusion, compared with the predicted rate. Relative ischemic stroke reduction compared with the predicted rate. (A) Overall patients. (B) Patients other than those with CHA2DS2-VASc score 0. Adapted from Kim et al. Front Cardiovasc Med 2022;9:853299, under the terms of the Creative Commons Attribution License (CC BY) [71].
Although more data on surgical LAAO are emerging, evidence from RCTs on LAAO remains scarce. The LAAOS III study (Left Atrial Appendage Occlusion Study III) randomized 4,811 patients who either underwent or did not undergo surgical LAAC at the time of cardiac surgery for another indication [72]. LAAC during cardiac surgery significantly decreased the risk of stroke/SE despite similar rates of remaining on long-term anticoagulation therapy. This RCT suggested that the combination of LAAC and long-term anticoagulation therapeutics can be beneficial and was therefore studied in patients with AF despite NOAC use.
Conclusions and future perspectives
Although anticoagulation with NOACs is the mainstay of AF management to prevent stroke, caution is required in patients with AF at high risk of stroke who have been under-represented or not studied in the RCTs of NOACs for stroke prevention. Development of acute ischemic stroke in patients with AF while receiving NOAC treatment is becoming an increasingly important issue in clinical practice. This is due to an increase in the number of patients with preceding NOAC therapy and a high risk of recurrence (>50% higher risk than in patients with AF and no prior anticoagulation therapy). Careful evaluation is mandatory to determine the possible causes of ischemic stroke during NOAC therapy (Table 1). Differentiation of AF-unrelated stroke and demonstration of combined cardiac diseases/systemic coagulopathy are important in these patients and may provide better results in their treatment. In addition, ensuring appropriate dosing and adherence to NOAC treatment is essential.
The results of the aforementioned studies showed that switching anticoagulation therapeutics does not reduce the risk of recurrent stroke in high-risk patients, including patients with renal impairment and older patients with multiple risk factors, and, more importantly, those who develop stroke on NOAC therapy. Novel pharmacological approaches, such as factor XIa inhibitors, and non-pharmacological interventional treatments, either percutaneous or surgical LAAO, are warranted in patients with AF and stroke despite sufficient NOAC use. Most recent guidelines recommend that LAAO may be considered for stroke prevention in patients with AF and contraindications for long-term anticoagulant treatment (e.g., intracranial bleeding without a reversible cause) and that surgical occlusion or exclusion of the LAA may be considered for stroke prevention in patients with AF undergoing cardiac surgery [5]. Although the current guidelines do not recommend interventional and surgical occlusions of the LAA in patients with NOAC failure, further studies are warranted to determine the role of strategies for occlusion or exclusion of LAA in these patients. The current ongoing trials regarding these issues are presented in Table 2.
Notes
Funding statement
This study was supported by the National Research Foundation of Korea grant (no. 2022R1A2C209148111).
Conflicts of interest
The authors have no financial conflicts of interest.
Author contribution
Conceptualization: OYB. Study design: OYB. Methodology: all authors. Data collection: all authors. Investigation: all authors. Writing—original draft: all authors. Writing—review & editing: all authors. Funding acquisition: OYB. Approval of final manuscript: all authors.


