Endovascular Treatment of Distal-M2 Segment Occlusions: A Clinical Registry and Meta-Analysis
Article information
Dear Sir:
Pending the results of randomized controlled trials (RCT) evaluating endovascular treatment (EVT) for distal occlusions, we aimed to investigate the safety and efficacy profiles of EVT for distal-M2 strokes in clinical practice.
We report data from the multicentric prospective registry ETIS (Endovascular Treatment for Ischemic Stroke) (ClinicalTrials.gov Identifier: NCT03776877) including 21 stroke centers in France. Our study received approval from our Intstitutionl Review Board (ID RCB 2017-A03457-46). Oral informed consent from the patient and/or a trustworthy person was collected by local investigators. We included patients with the following criteria: age ≥18 years; acute ischemic stroke (AIS) due to primary distal-M2 occlusion; and time from symptom onset to puncture ≤6 hours. An M2 occlusion was considered distal if it was above the horizontal line delineating the mid-height of the insula. The primary outcome was a favorable outcome, defined as a modified Rankin Scale (mRS) ≤2 at 90 days. Secondary outcomes included excellent outcome (mRS ≤1 at 90 days); 90-day all-cause mortality; early neurological improvement defined as a National Institutes of Health Stroke Scale (NIHSS) ≤1 or an improvement of ≥8 points compared with baseline at 24 hours post-EVT; procedure-related complications, including embolus to a new territory, perforation, and dissection; parenchymal hematoma (PH); symptomatic intracranial hemorrhage (sICH) defined as a hemorrhage on the follow-up computed tomography/magnetic resonance imaging scan and an increase of 4 points in the NIHSS score, according to the European Collaborative Acute Stroke Study classifications [1]; successful reperfusion defined as an improvement of at least 1 point in the modified Thrombolysis In Cerebral Infarction (mTICI) score between the first and the last angiogram (2a to 2b-2c-3, 2b to 2c-3). An additional systematic review with meta-analysis has been performed on distal-M2 thrombectomy and is detailed in Supplementary Material.
To identify outcome predictors in the present series, multiple regression models were fitted using the Akaike information criterion (AIC) and the Bayesian information criterion (BIC) [2]. Missing data were excluded. All analyses were completed using Stata 17 (StataCorp, College Station, TX, USA), RStudio version 1.4.1106 (https://posit.co/), and the R General Package for Meta-Analysis (version 6.0-0; R Foundation for Statistical Computing, Vienna, Austria).
During the study period, 157 patients underwent EVT for an AIS due to a distal-M2 occlusion. Demographic and procedural data are shown in Table 1. Median NIHSS at baseline was 12 (interquartile range [IQR]: 11). The median time between onset to puncture was 260 minutes (IQR: 130), and 84 patients (54.2%) received prior intravenous thrombolysis. Ninety-seven patients (63.8%) had a left distal-M2 occlusion.
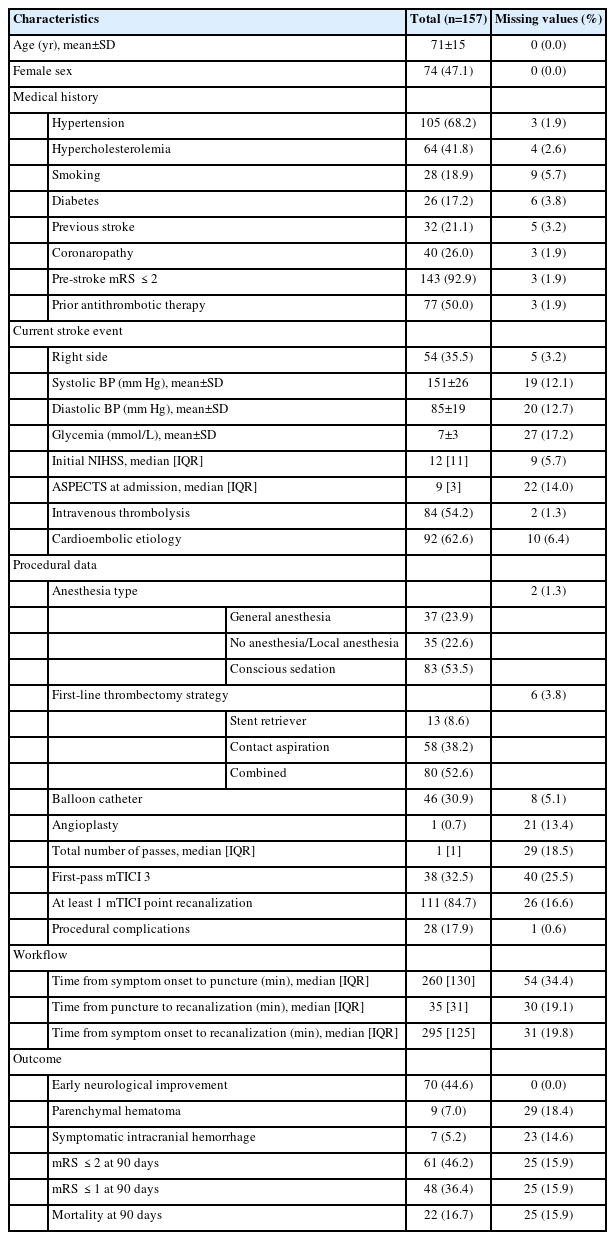
Baseline characteristics and outcomes of patients who underwent EVT for a distal-M2 occlusion in the ETIS cohort
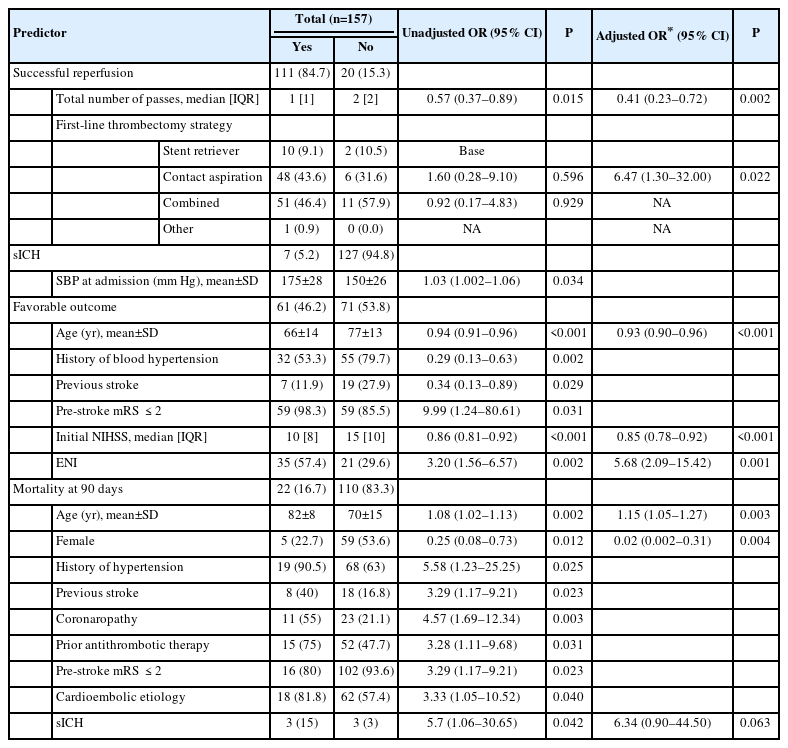
Successful reperfusion was achieved in 111 patients (84.7%), and 38 patients (32.5%) had an mTICI score of 3 after the first pass. In the multivariate analysis, successful reperfusion was associated with the total number of passes (odds ratio [OR] 0.41, 95% confidence interval [CI] 0.23–0.72, P=0.002) and the use of contact aspiration alone versus a stent retriever alone (OR 6.47, 95% CI 1.3–32, P=0.022). The later association was neither found amongst all M2 occlusions (i.e., proximal and distal) in the ETIS registry [3] nor in the ASTER (Contact Aspiration Versus Stent Retriever for Successful Revascularization) trial [4], and has to be cautiously interpreted. Atchaneeyasakul et al. [5] found that the utilization of a stent retriever in M2 occlusions resulted in more successful reperfusion compared to a pump aspiration system, but reperfusion outcomes were similar when comparing stent retriever to newer-generation pump aspiration catheter (e.g., Penumbra System ACE or MAX series). Also, the choice of first-line thrombectomy strategy was left at the discretion of the operator in our cohort. Larger prospective data comparing contact aspiration versus stent retrievers with the most recent devices are needed to confirm this association.
PH after EVT was seen in 9 patients (7.0%) and sICH was observed in 7 patients (5.2%). sICH was associated with a high systolic blood pressure at admission (OR 1.03, 95% CI 1.002–1.06, P=0.034). The rate of sICH in this series was lower to what was found on proximal-M2 occlusion in the ETIS registry reported by Muszynski et al. [3] (9.4%) and suggests that EVT for distal-M2 occlusions does not carry an additional risk of sICH.
Sixty-one patients (46.2%) achieved a favorable outcome. In the multivariate analysis, a favorable outcome was associated with age (OR 0.93, 95% CI 0.90–0.96, P<0.001), NIHSS at baseline (OR 0.85, 95% CI 0.78–0.92, P<0.001), and early neurological improvement (OR 5.68, 95% CI 2.09–15.42, P<0.001). Excellent outcome was observed in 48 patients (36.4%). Different factors could explain this low rate of favorable outcome for a distal occlusion: our large sample size (157 patients), a relatively low rate of intravenous thrombolysis in our series (54.2%), a longer time from symptom onset to treatment (260 min), and a high median NIHSS at baseline (12) with a large predominance of left distal-M2 occlusions (63.8%) supplying eloquent areas of the brain.
Mortality at 90 days was recorded in 22 patients (16.7%) and in the multivariate analysis was associated with age (OR 1.15, 95% CI 1.05–1.27, P=0.003) and female sex (OR 0.02, 95% CI 0.002–0.31, P=0.004). It suggests that AIS due to distal-M2 occlusions are often deadly, despite EVT and best medical treatment.
Predictors of successful reperfusion, sICH, favorable outcome, and mortality at 90 days are detailed in Table 2.
The strengths of our study are: (1) its prospective and multicentric design; (2) the large number of patients included compared to the previous series published on distal-M2 EVT; and (3) the systematic reporting of outcome data adopted by the participating centers of the ETIS registry. Nevertheless, the data drawn from the ETIS registry have some limitations: (1) the decision of thrombectomy was left at the discretion of the operator with a risk of selection bias, especially toward distal-M2 strokes that are more clinically severe, and outside the time window for intravenous thrombolysis; (2) no comparative group of distal-M2 strokes treated with the best medical treatment was available to assess the additional benefit of thrombectomy; and (3) it is limited to French stroke centers and might not be applicable to other populations.
In conclusion, EVT for distal-M2 occlusions resulted in a high rate of successful reperfusion and favored contact aspiration over the use of a stent retriever only in our study. RCTs are needed to demonstrate a potential benefit of EVT in addition to intravenous thrombolysis and identify the safest and most efficient first-line thrombectomy strategy in regard to this indication.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2022.03692.
Systematic review and meta-analysis on endovascular treatment for distal-M2 occlusions
Notes
Funding statement
None
Conflicts of interest
The authors have no financial conflicts of interest.
Author contribution
Conceptualization: SNF, BG, FC. Study design: SB, SNF, BL, BG, FC. Methodology: SB, SNF, BG, FC. Data collection: SNF, BL, GM, IS, SM, JMO, SR, CR, BG, FC. Investigation: SNF, BL, GM, IS, SM, JMO, SR, CR, BG, FC. Statistical analysis: SNF. Writing—original draft: SB, SNF. Writing—review & editing: SNF, BL, GM, IS, SM, JMO, SR, CR, BG, FC. Funding acquisition: BL, BG, FC. Approval of final manuscript: all authors.
Acknowledgements
List of ETIS Investigators is given in Appendix 1.
References
Appendices
Appendix 1.
Endovascular Treatment in Ischemic Stroke (ETIS) Investigators
jos-2022-03692-Appendix.pdf