Recanalization Therapies for Large Vessel Occlusion Due to Cervical Artery Dissection: A Cohort Study of the EVA-TRISP Collaboration
Article information
Abstract
Background and Purpose
This study aimed to investigate the effect of endovascular treatment (EVT, with or without intravenous thrombolysis [IVT]) versus IVT alone on outcomes in patients with acute ischemic stroke (AIS) and intracranial large vessel occlusion (LVO) attributable to cervical artery dissection (CeAD).
Methods
This multinational cohort study was conducted based on prospectively collected data from the EVA-TRISP (EndoVAscular treatment and ThRombolysis for Ischemic Stroke Patients) collaboration. Consecutive patients (2015–2019) with AIS-LVO attributable to CeAD treated with EVT and/or IVT were included. Primary outcome measures were (1) favorable 3-month outcome (modified Rankin Scale score 0–2) and (2) complete recanalization (thrombolysis in cerebral infarction scale 2b/3). Odds ratios with 95% confidence intervals (OR [95% CI]) from logistic regression models were calculated (unadjusted, adjusted). Secondary analyses were performed in the patients with LVO in the anterior circulation (LVOant) including propensity score matching.
Results
Among 290 patients, 222 (76.6%) had EVT and 68 (23.4%) IVT alone. EVT-treated patients had more severe strokes (National Institutes of Health Stroke Scale score, median [interquartile range]: 14 [10–19] vs. 4 [2–7], P<0.001). The frequency of favorable 3-month outcome did not differ significantly between both groups (EVT: 64.0% vs. IVT: 86.8%; ORadjusted 0.56 [0.24–1.32]). EVT was associated with higher rates of recanalization (80.5% vs. 40.7%; ORadjusted 8.85 [4.28–18.29]) compared to IVT. All secondary analyses showed higher recanalization rates in the EVT-group, which however never translated into better functional outcome rates compared to the IVT-group.
Conclusion
We observed no signal of superiority of EVT over IVT regarding functional outcome in CeAD-patients with AIS and LVO despite higher rates of complete recanalization with EVT. Whether pathophysiological CeAD-characteristics or their younger age might explain this observation deserves further research.
Introduction
Cervical artery dissection (CeAD) is a leading cause of ischemic stroke in the young (<50 years) [1]. Arterial embolism originating from the site of dissection, potentially leading to intracranial large vessel occlusion (LVO) is thought to be the primary mechanism for acute ischemic stroke (AIS) in CeAD-patients [2].
For AIS patients with LVO in the anterior circulation, multiple randomized-controlled trials (RCTs) have demonstrated superiority of endovascular treatment (EVT, with or without intravenous thrombolysis [IVT]) over IVT alone [3-7]. More recently, encouraging evidence has emerged also for EVT for basilary artery occlusion [8,9]. For CeAD-patients, however, observational studies failed to show superiority of EVT over IVT regarding clinical outcomes [2,10]. This might have technical and procedural grounds as most existing data on EVT in CeAD-patients were collected before 2015 [10] and thus before the major advances in EVT therapy. Even so, in a meta-analysis of the pivotal EVT-RCTs using current EVT standards, no treatment effect of EVT (compared to IVT) on clinical outcome at 3 months was shown for the subgroup of patients aged <50 years [11] in whom CeAD is a major cause of stroke. Although no specific data on CeAD-patients were provided, this finding might indicate that—even if current EVT standards are used—EVT may not be superior to IVT in AIS-LVO with underlying CeAD.
With these considerations in mind, we aimed to investigate the effect of EVT (with or without IVT) versus IVT alone on outcomes in CeAD-patients with LVO (due to thrombus embolization from the dissection) treated since 2015 in participating centers of the EVA-TRISP (EndoVAscular treatment and ThRombolysis in Ischemic Stroke Patients) collaboration.
Methods
Study design, study population, and study data
This study is based on prospectively collected data from the EVA-TRISP collaboration. The methods of EVA-TRISP have been described recently [12]. In brief, EVA-TRISP has evolved from the investigator-initiated, international ThRombolysis in Ischemic Stroke Patients (TRISP) collaboration [13], which published multiple international, multicenter studies on IVT in AIS patients and has now transformed to additionally include data on EVT-treated patients. As done in prior analyses, all data for the present study were collected locally in participating centers, anonymized, centrally pooled, and analyzed at the coordinating stroke center in Basel, Switzerland [14,15].
We included data from all CeAD-patients (including both internal carotid artery dissection [ICAD] and vertebral artery dissection [VAD]) with intracranial LVO proven by computed tomography angiography (CTA), magnetic resonance angiography (MRA), or digital subtraction angiography (DSA) who had either EVT (with or without IVT, henceforward referred to as “EVT”) or IVT alone (henceforward referred to as “IVT”) between January 1, 2015 and December 31, 2019. LVO was defined as an occlusion of the intracranial internal carotid artery, M1 and proximal M2 segment of the middle cerebral artery, A1 segment of the anterior cerebral artery, basilar artery, or the V4 segment of the vertebral artery. As done in prior research, CeAD was defined according to widely accepted imaging criteria: presence of a mural hematoma, aneurysmal dilatation, long tapering stenosis, intimal flap, double lumen, or occlusion situated >2 cm above the carotid bifurcation revealing an aneurysmal dilatation or a long tapering stenosis after recanalization [16]. Patients with purely intracranial dissection (e.g., V4 segment of the vertebral artery, petro-cavernous or intracranial ICAD, or dissection of the middle cerebral artery) were not included in the study. Patients with extracranial dissection with extension to intracranial segments of the artery (e.g., V2-/V3 segments of the vertebral artery with extension into the V4 segment of the dissected artery) were eligible for inclusion. CeAD-patients receiving neither EVT nor IVT are not included in EVA-TRISP and thus were not included in the current analyses. Patients with missing data on the primary clinical outcome measure were excluded.
All variables derived from the EVA-TRISP database for the present study are displayed in the results tables and were defined as in prior analyses [14,17]. In brief, these included (1) baseline demographic data (e.g., age, sex, and pre-stroke independency [prestroke modified Rankin Scale, mRS 0–2]), (2) medical history including vascular risk factors, (3) vital signs and baseline laboratory results, (4) information on the index event (i.e., clinical characteristics including stroke severity as measured by the National Institutes of Health Stroke Scale [NIHSS] score [18] on admission and at 24 hours) and radiological characteristics (i.e., site of intracranial occlusion and presence or absence of early ischemic changes), and (5) outcome data as specified below (study outcomes).
Regarding EVT- and IVT-procedures, the following time-based parameters were calculated: (1) onset-to-needle time (median, in minutes) defined as time from stroke onset to treatment initiation of IVT in IVT-treated patients or EVT-treated patients who had bridging therapy; (2) onset-to-groin time (median, in minutes) defined as time from stroke onset to groin puncture in EVTtreated patients.
Treatment
The choice of specific revascularization procedures was left to the discretion of the treating physicians in the participating centers taking respective guidelines as well as indication and contraindications of the respective treatments into account. EVT included the possible use of a stent retriever, mechanical aspiration, balloon angioplasty, and deposition of a permanent intracranial or extracranial stent, all as a single intervention or as a combination of different techniques as outlined in the EVA-TRISP-methods publication [12].
Study outcomes
The primary clinical outcome measure was a favorable 3-month functional outcome (i.e., mRS score 0–2). The primary radiological outcome measure was defined as successful arterial recanalization (i.e., thrombolysis in cerebral infarction [TICI] scale 2b/3) on first follow-up imaging [19] (i.e., CTA, MRA, DSA, or ultrasound [in the IVT-group]).
Secondary outcomes were defined as follows: (1) early neurological improvement (i.e., ≥50% improvement in NIHSS from admission to 24 hours; ΔNIHSS), (2) occurrence of symptomatic intracranial hemorrhage (sICH) according to the definition used in the Second European-Australasian Acute Stroke Study (ECASS II) [20], (3) any ICH, and (4) death.
Statistical analyses
Primary analyses
Baseline characteristics
We compared patients who had received EVT to those who had received IVT regarding baseline demographics, clinical, stroke, and stroke treatment variables using the chi-square test or the Fisher’s exact test (if appropriate) for categorical variables and the MannWhitney test for continuous variables. We calculated unadjusted odds ratios with 95% confidence intervals (OR [95% CI]) and respective P-values.
Primary and secondary outcome analyses
The association of EVT and IVT with primary and secondary outcome measures was assessed using a binary logistic regression model with calculation of unadjusted and adjusted (for age, sex, and NIHSS at admission) OR with 95% CI and respective P-values. For all analyses, a P-value less than 0.05 was considered statistically significant.
Secondary analyses
Analyses in patients with LVO in the anterior circulation (LVOant)
EVT in AIS with LVO was mainly studied and thus far was only shown superior to IVT in patients with LVOant. Thus, we focused our secondary analyses on patients with LVOant. We compared baseline characteristics in patients with LVOant who had received EVT to those who had received IVT using the same methodology as described for the primary analyses.
Propensity score matching
To reduce potential influence of between-group differences in key baseline variables on outcome analyses, we matched patients on their propensity for receiving EVT versus IVT. The propensity score was based on a multivariable logistic regression model with treatment allocation as the outcome variable and age, sex, and NIHSS at admission as independent variables. Patients were then matched in a 1:1 ratio with nearest-neighbor matching within a caliper of 0.2 standard deviations of the propensity score. We report unadjusted comparisons of important baseline characteristics in matched EVT and IVT patients with calculation of respective standardized mean differences. We further performed unadjusted logistic regression analyses in the matched dataset to assess associations of EVT and IVT with the primary study outcomes.
All analyses were performed using SPSS version 25 (IBM Corp., Armonk, NY, USA). The propensity score matching (PSM) analyses were performed using “R” version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
Ethics
The present study was approved by the ethics committee in Basel, Switzerland (KENZ; Ethikkommission Nordwest- und Zentralschweiz). The requirement for additional local ethics approval differed between participating centers; accordingly, approval was obtained if required. Informed consent was obtained if not waived by the respective authorities in participating centers.
Data availability
Datasets generated or analyzed within the present study are available from the corresponding author upon reasonable request. In each such case, compliance of data sharing with individual processes of patient consenting in participating centers is required. Final decision on data sharing will be made by consensus of the EVA-TRISP collaborators.
STROBE statement
A Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement checklist report on this study is available as supplementary material (Appendix 1).
Results
Primary analyses
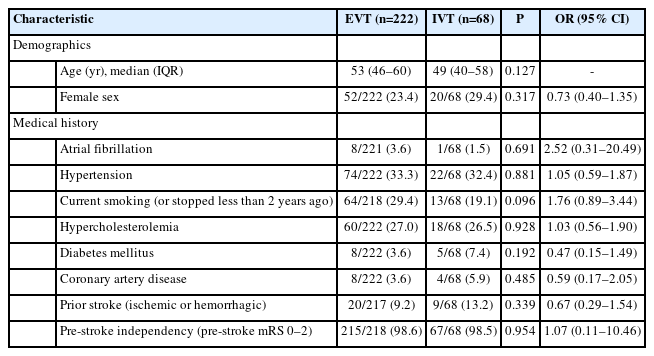
Of 369 CeAD-patients included in EVA-TRISP between 2015– 2019, 290 (78.6%) met the eligibility criteria and were included in the analyses. Main reasons for exclusion were the absence of LVO (n=36, 9.8%) and missing baseline or follow-up information (n=43, 11.7%). Details are given in the study flowchart (Figure 1). Of 290 patients included in the final analyses, 222 patients (76.6%) received EVT and 68 (23.4%) received IVT (Table 1).

Flowchart of included and excluded patients. CeAD, cervical artery dissection; EVA-TRISP, EndoVAscular treatment and ThRombolysis in Ischemic Stroke Patients; LVO, large vessel occlusion; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale score; IVT, intravenous thrombolysis; EVT, endovascular treatment.
Baseline characteristics
Patients in the EVT-group differed from those in the IVT-group in a higher median NIHSS at admission (14 [interquartile range, IQR 10–19] vs. 4 [IQR 2–7]) and in a higher rate of patients with wake-up stroke (17.4% vs. 2.9%) (Table 2). LVO in the anterior circulation was more frequent in the EVT-group (89.2% vs. 51.5%). Age, sex, medical history, baseline vital signs as well as laboratory results did not differ significantly between both treatment groups (Table 1).
In the EVT-group, 153 of 222 (68.9%) patients received IVT bridging therapy. Patients who had bridging therapy had significantly lower onset-to-needle times compared to patients who received IVT alone (108 vs. 139 minutes) (Table 2).
Primary clinical outcome measure
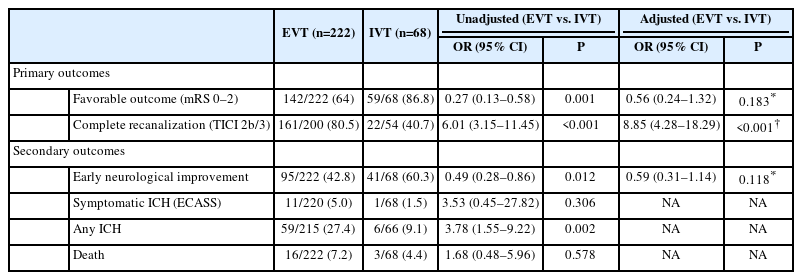
Favorable 3-month functional outcome was less frequent in the EVT-group (64.0%) compared to the IVT-group (86.6%), yielding an unadjusted OR of 0.27 (95% CI 0.13–0.58, P=0.001) (Table 3). This difference was no longer significant after adjustment for age, sex, and NIHSS at admission (ORadjusted 0.56 [95% CI 0.24–1.32], P=0.183).
Primary radiological outcome measure
Complete recanalization was more frequent in the EVT-group (80.5% vs. 40.7%) and the probability of complete recanalization was independently higher in the EVT-group (ORadjusted 8.85 [95% CI 4.28–18.29], P<0.001) (Table 3).
Secondary outcomes
Early neurological improvement occurred less frequently in the EVT-group (42.8%) as compared to the IVT-group (60.3%), yielding an unadjusted OR of 0.49 (95% CI 0.28–0.86, P=0.012). After adjustment for age, sex, and NIHSS at admission, this difference was no longer significant (ORadjusted 0.59 [95% CI 0.31–1.14], P=0.118).
Overall, 12 (of 290, 4.1%) sICH and 19 (of 290, 6.6%) deaths occurred. Thereby, both sICH and death were numerically more frequent in the EVT-group. This difference was not statistically significant in unadjusted analyses (Table 3). In the EVT-group, 7 of the 11 (63.6%) patients who had sICH, had received additional IVT.
Secondary analyses
Analyses in patients with LVO in the anterior circulation LVOant was present in 233 of 290 (80.3%) patients of whom 198 had received EVT and 35 had received IVT (Table 2 and Supplementary Table 1). Baseline characteristics in EVT- and IVT-treated patients with LVOant were comparable except for a higher median NIHSS (14.5 vs. 5, P<0.001) and higher median glucoseand CRP-levels in the EVT-group and a lower rate of patients with prior stroke in the EVT-group (Supplementary Table 1). In turn, median onset-to-needle time was higher in the IVT-group. In patients with LVOant, favorable 3-month outcome was significantly less frequent in EVT-treated than in IVT-treated patients (91.4% vs. 64.1%). After adjustment for age, sex, and baseline NIHSS, the probability of favorable 3-month outcome was lower for patients treated with EVT (ORadjusted 0.93 [95% CI 0.89–0.98]). The frequency and adjusted odds for complete recanalization were higher in the EVT-group (78.5% vs. 52.0%; ORadjusted 4.17 [95% CI 1.66–10.45]).
PSM analyses
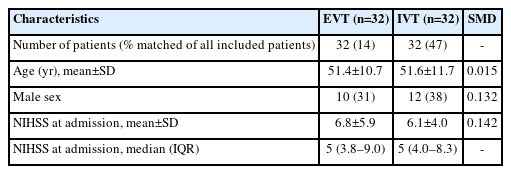
PSM analyses in patients with LVOant were based on 32 patients each from the EVT- and the IVT-group. Patient baseline characteristics of the matched variables (including NIHSS at admission) were comparable (Table 4). Unadjusted logistic regression analyses performed on the matched sample of patients with LVOant yielded lower odds for a favorable 3-month functional outcome in the EVT-group (OR 0.20 [95% CI 0.04–0.72]). The odds for complete recanalization were higher in the EVT-group (OR 2.2 [95% CI 0.86–9.36]); however—contrary to the primary and unmatched secondary analyses—this was no longer statistically significant.
Discussion
In this multinational analysis comparing EVT to IVT in CeAD-patients with LVO, there was no signal of superiority of EVT over IVT even if the higher stroke severity is considered and despite higher rates of complete recanalization in the EVT-group.
Considering the clear results from multiple RCTs demonstrating superiority of EVT compared to IVT alone in AIS with LVO in the general stroke population, we had assumed that CeAD-patients treated in this new EVT-era would benefit similarly. Unexpectedly, this hypothesis is not supported by our findings.
In contrast to our findings on clinical outcome, recanalization rates in the EVT-group in our study were significantly higher than in the IVT-group. This finding was consistent across all analyses we performed, indicating its robustness. Such discrepancy between favorable recanalization rates, yet lack of superiority in clinical outcomes was also seen to a similar extent in a prior study comparing EVT to IVT in CeAD patients [21]. Moreover, the rate of complete recanalization (TICI 2b/3) in the EVT-group in our study was comparable [5] to even higher [4,6] when compared to EVT-treated patients in the pivotal EVT-RCTs, thus proving technical success is achievable in EVT treatment of CeAD-patients as well. Nevertheless, this high recanalization rate did not translate into a better functional outcome of the EVT-group compared to the IVT-group, even if outcome predictive variables and in particular stroke severity were considered by different statistical means. Thus, further reasons for lack of (clinical) superiority of EVT over IVT in CeAD-patients must be explored.
The knowledge about the impact of EVT versus IVT on outcomes in CeAD-patients is currently based on few observational studies [10,21] while RCT-based comparative data is lacking. A recent meta-analysis across 14 observational studies and case series—most of which were published before 2015—showed no statistically significant difference between the EVT- and the IVT-group regarding favorable functional outcome in CeAD-patients [10].
Interestingly, in an age-dependent subgroup analysis of HERMES [Highly Effective Reperfusion Using Multiple Endovascular Devices], the group of stroke patients aged <50 years—in which CeAD is a leading stroke cause—was the only age-group that did not benefit from EVT [11]. Although information about stroke etiology was not available in this analysis, this observation might suggest that treatment effects of EVT in CeAD may be inferior compared to EVT in the general stroke population.
It is possible that the lack of superiority of EVT in our study is a spurious finding or—at least partly—due to yet undetected differences in baseline characteristics. We accounted for stroke severity, age, and sex and used PSM to minimize the effect of confounders; however, there might be other factors that we could not account for.
EVT might be technically more challenging in CeAD with presence of intimal tearing and a double lumen. However, this assumption is not supported by data from the interventional arm of the MR CLEAN (Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands) (n=15 CeAD-patients) trial as well as the MR CLEAN registry (n=59) comparing outcomes in AIS-LVO patients with CeAD to those with atherosclerotic lesions, which indicated a more favorable short-term neurological course in CeAD-patients [22]. In another study, clinical or procedural outcomes after thrombectomy in stroke patients with tandem lesions (i.e., intracranial artery occlusion and proximal stenosis or occlusion of the carotid artery) did not differ significantly between patients with CeAD (n=65) and those with atherosclerotic lesions (n=230) [23].
Alternatively, other aspects, which are independent from CeAD, but which are age-dependent might matter. This includes the role of collateral status and brain plasticity [24,25]. In younger stroke patients, an excellent collateral status and good brain plasticity could lower the relevance of recanalization of LVO on outcomes. In line with this assumption, studies comparing EVT versus IVT in AIS patients with LVO and mild symptoms (NIHSS ≤5)—which are likely to have excellent collaterals—EVT did not perform better than IVT despite higher rates of complete recanalization with EVT [26,27]. Interestingly, and probably supportive of this hypothesis, in an observational study performed by Yeo et al. [28] presenting outcomes of EVT in stroke patients aged <50 years and with various non-atherosclerotic stroke causes particularly prevalent in young patients, rates of favorable functional outcome varied between 57.1% and 71.4%—numbers comparing well with the outcomes seen in the EVT-group in our study.
The imbalance of LVO involving the anterior versus the posterior circulation was another potential confounder, as superiority of EVT over IVT in stroke in general is more established for LVO in the anterior than in the posterior circulation [8,9,29]. Betweengroup differences in favorable outcome became more pronounced (and in part statistically significant) in the subgroup of patients with LVOant. Though, conclusions from subgroup analysis may be misleading, they may support the idea that EVT might indeed be less effective in CeAD-patients in the general stroke patient population. These findings need further investigation.
In our study, sICH occurred numerically more often in the EVT-group, without reaching statistical significance. The overall rate of sICH in our EVT-group (5.0%) as well as the rate of sICH in patients who had had EVT with prior IVT (4.6%), however, is comparable to or even lower (when comparing patients without bridging) than in prior observational analyses investigating EVT in CeAD-patients [21,30]. More importantly, the sICH rate in our study is comparable to sICH rates in the pivotal randomized RCTs [3-7] indicating that EVT using current technologies seems relatively safe also in CeAD-patients regarding the occurrence of sICH.
We are aware of limitations of our study. First, treatment allocation was not randomized leading to an imbalance with higher stroke severity and predominant involvement of the anterior circulation in particular the carotid-T-occlusions in the EVT-group. Our means to counter such imbalances—including analyses focusing on patients with LVOant and the use of PSM—did not suggest a signal of clinical superiority of EVT over IVT in CeAD but came up to their limits and did not fully eliminate between-group differences. Thus, we urge a cautious interpretation of our key findings. This is particularly important for the analyses based on PSM, which led to comparable stroke severity in both groups but skewed the comparisons towards patients with less severe strokes. Further, other unknown or unmeasured confounders might be present and were not adjusted for. Second, given the design of our study, confounding by indication is likely. Particularly in patients with vertebral artery occlusion necessity to perform EVT depends on factors that we have not been able to account for (e.g., occlusion of the dominant vs. hypoplastic artery) but are likely to have influenced the decision to perform EVT or IVT or both. Third, despite being the largest cohort investigating EVT compared to IVT in CeAD to date, our sample size is still limited increasing the risk for spurious findings. Fourth, comparability of the primary radiological outcome (complete recanalization on first arterial imaging post-intervention in each group) can be debated, as an important factor—namely the very exact timepoint of recanalization—can rarely be assessed in patients receiving IVT. Fifth, except for the presence of early ischemic changes on baseline imaging, other important imaging variables which are likely associated with outcomes (i.e., Alberta Stroke Program Early Computed Tomography Score [ASPECTS], infarct volume on perfusion imaging, and collateral status) were not routinely assessed in the present dataset and therefore not included in the statistical models.
We are aware that our key observations are in dissent to both clinical expectations and guidelines about the use of EVT in LVO in general. Therefore, our findings should not be interpreted as argument to refrain from EVT in CeAD patients in clinical routine. Still, our unexpected results are nevertheless worth being made publicly known and should stimulate future research about why some LVO-patients might benefit less than expected from EVT and why this might be particularly the case in CeAD patients.
Our study has several strengths. The multicentric and multinational approach of the EVA-TRISP collaboration and the presented data ensure comparability of the data across centers and countries. Data in EVA-TRISP are collected prospectively including consecutive patients treated at the respective centers, thus reducing the risks of selection or inclusion bias.
Conclusions
Despite higher rates of complete recanalization, EVT in CeAD patients with AIS and LVO did not result in improved clinical outcomes when compared to IVT alone. This applied particularly for patients with LVO in the anterior circulation, even when matched for important clinical and outcome predictive variables. Whether pathophysiological characteristics of CeAD with potential technical implications for EVT in CeAD or particularities of stroke in younger patients might explain our observations deserve further research. The persisting conundrum of the use of EVT in CeAD patients would ideally be clarified in a randomized clinical trial, of which feasibility is questionable. Reassuringly, however, our study did not produce evidence, that EVT in these patients is clinically harmful. Thus, EVT should not be withheld in CeAD-patients in clinical practice.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2022.03370.
Baseline, clinical, and stroke characteristics in patients with LVO in the anterior circulation
Notes
Funding statement
None
Conflicts of interest
The disclosures of conflict of interest of all authors are provided in Appendix 2.
Author contribution
Conceptualization: CT, STE, HG. Study design: CT, STE, HG. Methodology: CT, STE, HG. Data collection: all authors. Investigation: all authors. Statistical analysis: CT, JL, HG. Writing—original draft: CT, STE, HG. Writing—review & editing: all authors. Approval of final manuscript: all authors.