 |
 |
- Search
| J Stroke > Volume 25(2); 2023 > Article |
|
Abstract
Background and Purpose
Randomized trials proved the benefits of mechanical thrombectomy (MT) for select patients with large vessel occlusion (LVO) within 24 hours of last-known-well (LKW). Recent data suggest that LVO patients may benefit from MT beyond 24 hours. This study reports the safety and outcomes of MT beyond 24 hours of LKW compared to standard medical therapy (SMT).
Methods
This is a retrospective analysis of LVO patients presented to 11 comprehensive stroke centers in the United States beyond 24 hours from LKW between January 2015 and December 2021. We assessed 90-day outcomes using the modified Rankin Scale (mRS).
Results
Of 334 patients presented with LVO beyond 24 hours, 64% received MT and 36% received SMT only. Patients who received MT were older (67±15 vs. 64±15 years, P=0.047) and had a higher baseline National Institutes of Health Stroke Scale (NIHSS; 16±7 vs.10±9, P<0.001). Successful recanalization (modified thrombolysis in cerebral infarction score 2b-3) was achieved in 83%, and 5.6% had symptomatic intracranial hemorrhage compared to 2.5% in the SMT group (P=0.19). MT was associated with mRS 0-2 at 90 days (adjusted odds ratio [aOR] 5.73, P=0.026), less mortality (34% vs. 63%, P<0.001), and better discharge NIHSS (P<0.001) compared to SMT in patients with baseline NIHSS ≥6. This treatment benefit remained after matching both groups. Age (aOR 0.94, P<0.001), baseline NIHSS (aOR 0.91, P=0.017), Alberta Stroke Program Early Computed Tomography (ASPECTS) score ≥8 (aOR 3.06, P=0.041), and collaterals scores (aOR 1.41, P=0.027) were associated with 90-day functional independence.
Large vessel occlusion (LVO) accounts for 30% of acute ischemic stroke (AIS) and is associated with high stroke-related death and disability. Mechanical thrombectomy (MT) has proven benefits in select LVO patients within 24 hours from last-known-well (LKW) with a number needed to treat as low as 2.8 to achieve functional independence [1,2]. Real-world data of patients treated with MT mirrors the trial outcomes, with satisfactory procedural and clinical outcomes [3].
Several studies have reported that some LVO patients may have persistent salvageable tissue beyond 24 hours of LKW [4-6]. Factors including ischemia severity and the robustness of cerebral collateral circulation determine this slow infarct progression rate [7]. These “slow progressors” might still benefit from MT [8].
A randomized controlled trial to investigate the effectiveness and safety of MT beyond 24 hours from LKW would be ideal but challenging due to a potentially low number of LVO patients with a favorable clinical imaging profile at this time window [9]. This study reports our cumulative experience with MT beyond the current guidelines-based treatment window and provides preliminary data on MT safety and outcomes beyond 24 hours.
This is a retrospective analysis of acute LVO patients who presented to 11 comprehensive stroke centers in the United States beyond 24 hours from LKW and were treated with MT between January 2015 and December 2021. Data on LVO patients presented during the same period and treated with standard medical therapy (SMT) alone were available from 5 of the participating centers. All data were collected retrospectively from the endovascular databases maintained by each participating center investigators and approved by local institutional review boards. Informed consent was waived because this was a retrospective analysis.
All patients received baseline non-contrast computed tomography (NCCT) and computed tomography angiography (CTA) at presentation. Perfusion imaging for patient selection was performed depending on the local institutional standards of care. Demographic, clinical, imaging, procedural variables, in-hospital complications, and outcomes at 90 days were analyzed. Stroke severity was measured by the National Institutes of Health Stroke Scale (NIHSS). Alberta Stroke Program Early Computed Tomography Score (ASPECTS) and the posterior circulation ASPECTS (pc-ASPECTS) were used for infarct volume estimate on initial NCCT in the anterior circulation and posterior circulation [10]. Collateral circulation was quantified using the CTA collateral score on single-phase CTA images: scores range from 0 indicating absent collaterals in >50% of the middle cerebral artery (MCA) M2 branch territories to 4 indicating robust collaterals [11]. A vascular neurologist or neuroradiologist reviewed all imaging data independently in each institution. Successful reperfusion was defined as modified thrombolysis in cerebral infarction (mTICI) score 2b-3. Stroke etiology was classified based on the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria [12]. Postprocedural intracranial hemorrhage (ICH) was defined using the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST) classification [13]. Neurological outcomes were measured using the modified Rankin Scale (mRS) at 90 days.
The primary outcome was the rate of functional independence defined as mRS 0-2 at 90 days in the MT group compared to the SMT group. Safety outcomes were mortality rates at 90 days and symptomatic ICH (sICH) defined as PH2 in the same arterial territory resulting in NIHSS decline of ≥4 points in both MT and SMT groups. Secondary outcomes were rates of successful recanalization in the MT group, discharge NIHSS scores, shift towards a better functional outcome (mRS) at 90 days, and rates of hospital complications (cerebral edema with midline shift in the same arterial territory and/or hemicraniectomy) in both groups.
All continuous variables were reported as mean with standard deviation or median and interquartile range as appropriate and were analyzed using Wilcoxon rank-sum test. Categorical variables were reported as percentages and analyzed using Pearson’s chi-squared test. We investigated the univariable and multivariable correlations between functional outcomes and variables of interest as covariates. A binomial logistic regression model was used for estimating the probability of favorable functional outcomes (mRS 0-2) at 90 days. The distribution of 90-day mRS score analyses (ordinal shift) was analyzed using multinomial ordinal logistic regression; a shift to the value in the lower order was considered a better outcome. A subgroup analysis was also performed, including univariable and multivariable binomial and ordinal regression of patients with baseline NIHSS scores higher or equal to 6. For logistic regressions, crude and adjusted odds ratios (aOR) and 95% confidence intervals (CI) were reported for each parameter. To reduce the risk of selection bias, we conducted a propensity score-matched analysis for patients with NIHSS ≥6 to obtain comparable groups (mechanical thrombectomy and medical treatment) matching for baseline charectrestics including age, sex, race, hypertension, diabetes mellitus, congestive heart failure, coronary artery disease, hyperlipidemia, prior stroke, initial NIHSS, baseline ASPECTS score, baseline mRS, LVO location, tandem occlusion, and stroke etiology. Propensity scores for all patients were estimated, representing the probability of group assignment with respect to observed baseline covariates. We matched participants using a simple 1:1 nearest neighbor matching without replacement. A caliper of 0.20 of the standard deviation of the legit propensity score was imposed to obtain similar groups given the identified set of covariates. Standardized mean differences were examined to compare patient features before and after matching, with imbalance being defined as an absolute value greater than 0.20 (small effect size). P values less than 0.05 were considered significant and all tests were two-tailed. All statistical analysis was conducted using Easy R (EZR) package on R language software version 4.21 (R Foundation for Statistical Computing, Vienna, Austria) and jamovi open-source R-based statistical software version 2.3.18 (https://www.jamovi.org).
Three hundred thirty-four patients presented with LVO beyond 24 hours, 214 (64%) received MT and 120 (36%) were medically treated. Patients who received MT were older (64±15 vs. 67±15 years, P=0.047), had shorter LKW to presentation mean (50±39 vs. 77±67 hours, P<0.001), had higher initial NIHSS score (16±7 vs. 10±9, P<0.001) with NIHSS ≥6 in 95% compared to 50% in the SMT group (P<0.001), and more frequent tandem occlusions (14% vs. 5%, P=0.01). Atherosclerosis was the predominant stroke etiology in both groups (Table 1).
Successful recanalization (mTICI 2b-3) was achieved in 177 (83%) MT-treated patients (Table 1). Twelve (5.6%) had sICH compared to 3 (2.5%) in the SMT group (P=0.19). Functional outcomes at 90 days were available for 262 patients; there was no statistically significant difference in the discharge NIHSS (P=0.07), overall 90-day functional outcomes (P=0.2), or mortality (P=0.55) between both groups. However more patients in the SMT group achieved functional independence (mRS 0-2) at 90 days compared to the MT group (40% vs. 28%, P=0.04) (Table 2).
In the subgroup of patients with NIHSS ≥6, MT-treated patients had higher odds of achieving functional independence at 90 days (aOR 5.73, 95% CI [1.23-26.7], P=0.026), a significant shift towards a lower degree of functional disability at 90 days (aOR 4.65 [95% CI 1.95-11.69], P<0.001), less frequent hospital complications (cerebral edema and/or hemicraniectomy P<0.001), better discharge NIHSS (15±14 vs. 23±13, P<0.001), better 90-day overall mRS (3±2 vs. 4±2, P=0.001), and lower mortality (34% vs. 63%, P<0.001) compared to SMT (Table 3, Figures 1 and 2). MT treatment benefit was persistent for both anterior and posterior LVO (Supplementary Table 1 and 2). Age (aOR 0.94, 95% CI [0.91-0.97], P<0.001), baseline NIHSS (aOR 0.91, 95% CI [0.84-0.98], P=0.017), and ASPECTS score ≥8 (aOR 3.06 [95% CI 1.05-8.94], P=0.041) were associated with 90-day functional independence (Table 4). Additionally, the collaterals score (aOR 1.41, 95% CI [1.04, 1.92], P=0.027) was associated shift towards a lower degree of functional disability at 90 days (Supplementary Table 3). There was no significant effect of LKW, baseline mRS, or LVO location on 90-day outcomes.
Ninety-four patients (47 in each group) were matched. MT-treated patients had better discharge NIHSS (10 vs. 19, P=0.002) and lower mortality (24% vs. 55%, P=0.009). Although there was a numerically higher proportion with functional independence at 90 days (20% vs. 13%), this did not reach statistical significance (P=0.54) (Table 5).
In our cohort, 214 patients received MT up to 72 hours from LKW. These patients presented with more severe initial stroke severity compared to the medically treated patient yet had higher rates of functional independence, a shift towards lower degrees of functional disability at 90 days, and lower mortality. This approach is supported by recent studies that have reported that the core-penumbra mismatch profile could persist beyond 24 hours in patients with LVO [2]. Several smaller series have also reported that MT can be safely performed in selected patients with salvageable brain tissue beyond 24 hours [4,6,14].
Functional independence was achieved in 28% of the MT-treated group. Our results are comparable to other studies addressing MT beyond 24 hours [4,6,15] but lower than the reported rate in the randomized MT clinical trials of LVO <24 hours from LKW [2,16]. However, these patients represent a real-world experience and have presented at a much later time-window with potential infarct core size variation at presentation. Another contributing factor is the higher prevalence of large artery atherosclerotic (LAA) occlusion in this population compared to the overall LVO population (37%). Data from the German Stroke Registry-Endovascular Treatment have shown that patients with LVO due to LAA had worse outcomes than those with a cardioembolic etiology [17]. This higher prevalence of atherosclerotic occlusion may in fact account for the delayed presentation partly due to ischemic preconditioning, more robust collaterals, and longer resistance to ischemia in patients with atherosclerosis [18]. Lee et al. [19] found that the delayed treatment beyond 24 hours of LKW was significantly more common in patients with intracranial atherosclerotic occlusion (ICAS-O) than that in patients with cardioembolic occlusion, ICAS-O can begin with relatively mild or fluctuating symptoms due to better collaterals, and endovascular treatment is performed after the observation of worsening in the symptoms.
In our cohort, MT treatment effect over conservative SMT was predominant in patients who presented with more severe strokes. MT benefits in patients with LVO and minor stroke symptoms are controversial [20]; meta-analyses of pooled original data showed no benefit over SMT for patients with low NIHSS [21], and other studies have shown overall 90-day outcomes benefit favoring MT, especially in the subset that is likely to deteriorate due to more proximal occlusions [20,22,23]. These conflicting results may reflect heterogeneity in treatment decision selection criteria for this patient population beyond the initial NIHSS score.
The optimal imaging paradigm for patient selection in this very late window remains uncertain. In our cohort, good ASPECTS and collateral scores—surrogates for viable brain and slow infarct progression—were associated with good functional outcomes at 90 days. Multiple studies have addressed the reliability of NCCT to evaluate irreversible brain tissue injury and concluded that NCCT ASPECTS is comparable to advanced perfusion studies for MT-patient selection with similar procedural outcomes even in the extended treatment window [4,24-26]. Bouslama et al. [27] compared different imaging paradigms for MT patient selection and reported that the clinical-ASPECTS mismatch paradigm is simple, more inclusive, and more easily incorporated into clinical practice for MT compared to the perfusion-imaging mismatch paradigm. A recent study stratified patients presenting in the very late time window by the initial imaging modality (NCCT/CTA) only or perfusion-based imaging and showed no significant differences in functional and safety outcomes [4]. On the other hand, the AURORA (Analysis of Pooled Data from Randomized Studies of Thrombectomy More Than 6 Hours After Last Known Well) study investigators reported that patients with an undetermined mismatch profile (i.e., perfusion imaging not performed) did not experience a treatment benefit compared to patients who met the clinical or target perfusion mismatch profile [28].
A recent retrospective study of 185 patients with anterior circulation LVO treated with MT beyond 24 hours from LKW also showed the potential benefit of MT [15]. The majority of MT patients in that study (62%) had perfusion imaging. Compared to 116 SMT patients, those treated with MT had better functional outcomes (38% vs. 10%) and lower mortality (26% vs. 41%) but higher sICH risk (10.1% vs. 1.7%). Propensity score matching showed an association of favorable functional outcomes with MT but no differences in mortality and the benefits were similar whether patients were selected by perfusion imaging or by ASPECTS score. These results are consistent with our findings despite the differences in our populations: inclusion of posterior circulation strokes and higher stroke severity in our MT group compared to SMT.
Successful reperfusion was achieved in 83% of our cohort. This rate is comparable to the reperfusion rates of 84% in the DAWN (Thrombectomy 6-24 Hours after Stroke with a Mismatch Between Deficit and Infarct) and 76% in DEFUSE3 (Thrombectomy for Stroke at 6-16 Hours with Selection by Perfusion Imaging) clinical trials [6,28] and studies addressing MT beyond the DAWN paradigm (80%-81%) [4,14]. Importantly, revascularization in this delayed treatment window was not associated with an increase in sICH compared to the rates in patients treated under 24 hours despite a high revascularization rate and late treatment window [28]. This observed lower sICH rate of 5.6% compared to 10.1% observed in the study by Sarraj et al. [15] can be possibly due to differences in the patient populations; however, sICH rate did not overwhelm the potential benefit in functional outcomes.
To our knowledge, this is the largest multicenter cohort in North America addressing MT beyond the accepted 24-hour time-window. This study highlights the safety and efficacy of MT compared to SMT beyond the conventional 24 hours from LKW for LVO treatment. Our study has limitations inherent to retrospective studies including possible data entry errors and missing data especially 90-day functional outcomes. Secondly, there is the heterogeneity between the centers in the utilization of perfusion studies data for this patient population treatment selection and the limited available perfusion studies data in our cohort, which limited the analysis of outcome regarding the imaging modality selection criteria. Thirdly, the small numbers in the propensity-matched group limited achieving statistically significant results in MT group.
MT in selected patients was associated with higher odds of functional independence and better outcomes than SMT, predominately in patients with severe strokes. Younger age, good ASPECTS, and collateral scores were all associated with good functional outcomes. Although these findings need to be validated in a prospective study, it is reasonable to consider MT for patients presenting with LVO stroke who have evidence of tissue viability and resilience to ischemia regardless of stroke duration.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2023.00017.
Supplementary Table 1.
Outcomes by treatment modality: subgroup analysis of anterior circulation, NIHSS ≥6
Supplementary Table 2.
Outcomes by treatment modality: subgroup analysis of posterior circulation, NIHSS ≥6
Supplementary Table 3.
Predictors of functional outcomes (ordinal analysis): subgroup analysis of NIHSS ≥6 population
Notes
Author contribution
Conceptualization: GAM, ABC. Study design: GAM. Methodology: GAM. Data collection: all authors (except for MAE). Investigation: GAM. Statistical analysis: GAM, MAE. Writing—original draft: GAM, HAN, ABC. Writing—review & editing: all authors. Approval of final manuscript: all authors.
Figure 1.
Distribution of 90-day mRS scores by treatment modality: subgroup analysis of NIHSS ≥6 population. mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale.

Figure 2.
Good functional outcomes by treatment modality and initial NIHSS scores ≥6. (A) Medical therapy. (B) Mechanical thrombectomy. mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale.
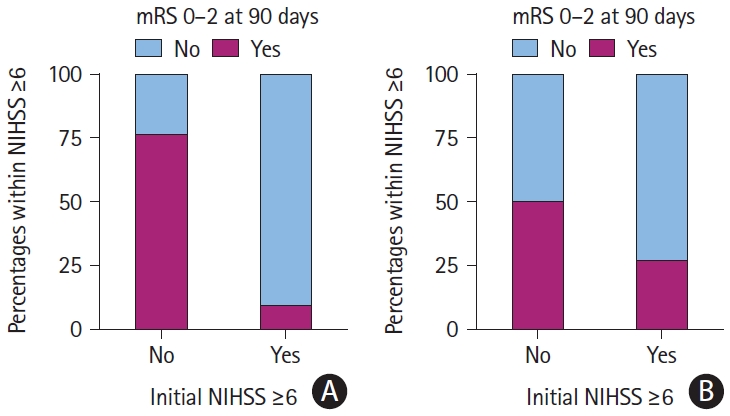
Table 1.
Baseline characteristics of MT and SMT group
Data are presented as mean±standard deviation (range) or n (%) unless otherwise indicated.
MT, mechanical thrombectomy; SMT, standard medical therapy; IQR, interquartile range; LSW, last seen well; BMI, body mass index; ED, emergency department; BP, blood pressure; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; NCCT, non-contrast computed tomography; MRI, magnetic resonance imaging; ASPECTS, Alberta Stroke Program Early Computed Tomography Score; CTA, computed tomography angiography; MRA, magnetic resonance angiography; ACA, anterior cerebral artery; PCA, posterior cerebral artery; VA, vertebral artery; BA, basilar artery; ICA, internal carotid artery; mTICI, modified thrombolysis in cerebral infarction; ESUS, embolic stroke of undetermined source.
Table 2.
Outcomes for MT and SMT groups
| SMT (n=120, 36%) | MT (n=214, 64%) | P | |
|---|---|---|---|
| Any ICH | 14 (12) | 55 (26) | 0.002 |
| sICH | 3 (2.5) | 12 (5.6) | 0.19 |
| Cerebral edema in same arterial territory with midline shift | 25 (20.8) | 26 (12.1) | 0.03 |
| Hemicraniectomy | 7 (5.8) | 8 (3.7) | 0.38 |
| Predischarge NIHSS | 14±15 (0-42) | 15±14 (0-42) | 0.07 |
| 90-day mRS* | 4±2 (0-6) | 4±2 (0-6) | 0.20 |
| Median (IQR) | 3 (1-6) | 4 (2-6) | 0.20 |
| mRS 0-2 at 90 days* | 34 (40) | 49 (28) | 0.04 |
| Mortality at 90 days* | 32 (38) | 61 (34) | 0.55 |
Data are presented as mean±standard deviation (range) or n (%) unless otherwise indicated.
MT, mechanical thrombectomy; SMT, standard medical therapy; ICH, intracranial hemorrhage; sICH, symptomatic intracranial hemorrhage; NIHSS, National Institutes of Health Stroke Scale; mRS, modified Rankin Scale; IQR, interquartle range.
Table 3.
Outcomes by treatment modality: subgroup analysis of NIHSS ≥6 population
| SMT (n=59, 22%) | MT (n=204, 78%) | P | |
|---|---|---|---|
| Any ICH | 13 (22.0) | 51 (25.0) | 0.64 |
| sICH | 3 (5.1) | 9 (4.4) | 0.73 |
| Classification of ICH | 0.43 | ||
| None | 49 (83.1) | 161 (78.9) | |
| HI1 | 2 (3.4) | 18 (8.8) | |
| HI2 | 5 (8.5) | 11 (5.4) | |
| PH1 | 0 (0) | 4 (2.0) | |
| PH2 | 3 (5.1) | 6 (2.9) | |
| SAH | 0 (0) | 4 (2.0) | |
| Cerebral edema in same arterial territory with midline shift | 20 (33.9) | 26 (12.7) | <0.001 |
| Hemicraniectomy | 6 (10.2) | 8 (3.9) | 0.09 |
| Predischarge NIHSS | 23±13 (1-42) | 15±14 (0-42) | <0.001 |
| 90-day mRS* | 4±2 (1-6) | 3±2 (0-6) | 0.001 |
| mRS 0-2 at 90 days* | 4 (9.3) | 47 (27) | 0.01 |
| Mortality at 90 days* | 27 (63) | 60 (34) | <0.001 |
Data are presented as mean±standard deviation (range) or n (%).
NIHSS, National Institutes of Health Stroke Scale; MT, mechanical thrombectomy; SMT, standard medical therapy; ICH, intracranial hemorrhage; sICH, symptomatic intracranial hemorrhage; HI1, hemorrhagic infarction type 1; HI2, hemorrhagic infarction type 2; PH1, parenchymal hematoma type 1; PH2, parenchymal hematoma type 2; SAH, subarachnoid hemorrhage; mRS, modified Rankin Scale.
Table 4.
Predictors of good functional outcomes (mRS 0-2): subgroup analysis of NIHSS ≥6
Table 5.
Propensity matched score
Data are presented as n (%) or median (interquartile range).
SMT, standard medical therapy; MT, mechanical thrombectomy; ICH, intracranial hemorrhage; sICH, symptomatic intracranial hemorrhage; HI1, hemorrhagic infarction type 1; HI2, hemorrhagic infarction type 2; PH1, parenchymal hematoma type 1; PH2, parenchymal hematoma type 2; SAH, subarachnoid hemorrhage; NIHSS, National Institutes of Health Stroke Scale; mRS, modified Rankin Scale.
References
1. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018;378:11-21.

2. Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, OrtegaGutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018;378:708-718.



3. Haussen DC, Al-Bayati AR, Mohammaden MH, Sheth SA, Salazar-Marioni S, Linfante I, et al. The Society of Vascular and Interventional Neurology (SVIN) mechanical thrombectomy registry: methods and primary results. Stroke Vasc Interv Neurol 2022;2:e000234.

4. Dhillon PS, Butt W, Podlasek A, Barrett E, McConachie N, Lenthall R, et al. Endovascular thrombectomy beyond 24 hours from ischemic stroke onset: a propensity score matched cohort study. J Neurointerv Surg 2023;15:233-237.


5. Abou-Chebl A. Endovascular treatment of acute ischemic stroke may be safely performed with no time window limit in appropriately selected patients. Stroke 2010;41:1996-2000.


6. Purrucker JC, Ringleb PA, Seker F, Potreck A, Nagel S, Schönenberger S, et al. Leaving the day behind: endovascular therapy beyond 24h in acute stroke of the anterior and posterior circulation. Ther Adv Neurol Disord 2022;15:17562864221101083.




7. Heiss WD, Rosner G. Functional recovery of cortical neurons as related to degree and duration of ischemia. Ann Neurol 1983;14:294-301.


8. Rocha M, Jovin TG. Fast versus slow progressors of infarct growth in large vessel occlusion stroke: clinical and research implications. Stroke 2018;48:2621-2627.

9. Lee KJ, Kim BJ, Kim DE, Ryu WS, Han MK, Kim JT, et al. Nationwide estimation of eligibility for endovascular thrombectomy based on the DAWN trial. J Stroke 2018;20:277-279.




10. Filep RC, Marginean L, Stoian A, Bajko Z. Diagnostic and prognostic computed tomography imaging markers in basilar artery occlusion (review). Exp Ther Med 2021;22:954.



11. Souza LC, Yoo AJ, Chaudhry ZA, Payabvash S, Kemmling A, Schaefer PW, et al. Malignant CTA collateral profile is highly specific for large admission DWI infarct core and poor outcome in acute stroke. AJNR Am J Neuroradiol 2012;33:1331-1336.



12. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35-41.


13. Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet 2007;369:275-282.


14. Desai SM, Haussen DC, Aghaebrahim A, Al-Bayati AR, Santos R, Nogueira RG, et al. Thrombectomy 24 hours after stroke: beyond DAWN. J Neurointerv Surg 2018;10:1039-1042.


15. Sarraj A, Kleinig TJ, Hassan AE, Portela PC, Ortega-Gutierrez S, Abraham MG, et al. Association of endovascular thrombectomy vs medical management with functional and safety outcomes in patients treated beyond 24 hours of last known well: The SELECT Late Study. JAMA Neurol 2023;80:172-182.


16. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018;378:11-21.

17. Tiedt S, Herzberg M, Küpper C, Feil K, Kellert L, Dorn F, et al. Stroke etiology modifies the effect of endovascular treatment in acute stroke. Stroke 2020;51:1014-1016.

18. Guglielmi V, LeCouffe NE, Zinkstok SM, Compagne KCJ, Eker R, Treurniet KM, et al. Collateral circulation and outcome in atherosclerotic versus cardioembolic cerebral large vessel occlusion. Stroke 2019;50:3360-3368.


19. Lee D, Lee DH, Suh DC, Kim BJ, Kwon SU, Kwon HS, et al. Endovascular treatment in patients with cerebral artery occlusion of three different etiologies. J Stroke 2020;22:234-244.




20. McCarthy DJ, Tonetti DA, Stone J, Starke RM, Narayanan S, Lang MJ, et al. More expansive horizons: a review of endovascular therapy for patients with low NIHSS scores. J Neurointerv Surg 2021;13:146-151.


21. Goyal N, Tsivgoulis G, Malhotra K, Ishfaq MF, Pandhi A, Frohler MT, et al. Medical management vs mechanical thrombectomy for mild strokes: an international multicenter study and systematic review and meta-analysis. JAMA Neurol 2020;77:16-24.


22. Haussen DC, Bouslama M, Grossberg JA, Anderson A, Belagage S, Frankel M, et al. Too good to intervene? Thrombectomy for large vessel occlusion strokes with minimal symptoms: an intention-to-treat analysis. J Neurointerv Surg 2017;9:917-921.


23. Sarraj A, Hassan A, Savitz SI, Grotta JC, Cai C, Parsha KN, et al. Endovascular thrombectomy for mild strokes: how low should we go? A multicenter cohort study. Stroke 2018;49:2398-2405.



24. Nguyen TN, Abdalkader M, Nagel S, Qureshi MM, Ribo M, Caparros F, et al. Noncontrast computed tomography vs computed tomography perfusion or magnetic resonance imaging selection in late presentation of stroke with large-vessel occlusion. JAMA Neurol 2022;79:22-31.


25. Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372:2296-2306.


26. Bouslama M, Haussen DC, Rodrigues G, Barreira C, Frankel M, Nogueira RG. Novel selection paradigms for endovascular stroke treatment in the extended time window. J Neurol Neurosurg Psychiatry 2021;92:1152-1157.










