Dear Sir:
Some degree of cognitive impairment (CI) is observed in at least half of stroke survivors, two-thirds corresponding to mild CI and a third to dementia [1]. Regardless of severity, poststroke CI has severe consequences on autonomy, institutionalization [2,3], quality of life [4], and the rate of recurrence of a major vascular event and death [5]. Although poststroke CI is classically attributed to vascular brain lesions, pioneer studies reported that this accounts for only half of poststroke dementia [6-8]. A third of poststroke dementia has been attributed to associated Alzheimer’s disease (AD) on clinical grounds [6-8]. With the development of amyloid positron emission tomography (PET), it is now possible to determine the presence of amyloid burden, a marker of AD, in poststroke CI. Four studies have assessed amyloid burden using PET following a stroke and reported a prevalence ranging from 10.5% to 22.2% in series of 26 to 81 patients [9-12]. Surprisingly only one study found a relationship with cognitive outcome at follow-up [9]. This wide range of prevalence and the questionable relationship with cognitive status raise serious questions concerning the previously reported contribution of AD to poststroke CI.
To address the contribution of amyloid burden to poststroke CI, we designed the IDEA3 (Imagerie des dépôts amyloïdes cérébraux par florbetapir [AV-45] et diagnostic des déficits cognitifs et démence post Accident Vasculaire Cérébral) study which assessed the amyloid PET status in stroke patients with some degree of CI and their outcome at 5 years. The main objective of the present study was to determine the frequency of amyloid PET positivity, and the secondary objective was to determine the relationship with outcome at inclusion (i.e., at time of PET examination).
The inclusion criteria were similar to those of GRECogVASC (Groupe de Réflexion pour l’Évaluation Cognitive Vasculaire) study [13]. Briefly, eligible patients had to be hospitalized in our center for an acute (<30 days) ischemic or hemorrhagic stroke with positive imaging, to have at least one impaired cognitive score at the follow-up assessment, and to be free of exclusion criteria (Supplementary Method 1). The main exclusion criteria included known conditions (other than stroke) that affect cognition and large cerebellar lesions.
From September 2010 to February 2018, 91 patients were included in IDEA3 (Supplementary Figure 1). Infarcts were observed for 81 patients (89%); the 10 (11%) cerebral hemorrhages were mainly due to hypertension and cerebral amyloid angiopathy (Table 1). A group of 1,003 healthy volunteers were used to analyze the cognitive data [14]. The study was performed in accordance with institutional guidelines and was approved by the regional investigational review board (Comité de Protection de Personnes Nord-Ouest II, Amiens, France; reference: 2013/27, July 11, 2013) and informed consent was obtained from patients.
The screening visit was scheduled at 6 months poststroke and included clinical and cognitive assessments. Patients fitting the inclusion criteria and consenting to participate were then planned for the amyloid PET exam. The supply of florbetapir determined the date of the inclusion visit (M0), ideally scheduled in the first year after the stroke. However, severe supply delays led to later inclusion of the first patients. The inclusion visit corresponded to the day of the PET examination and included clinical, neuropsychological, and magnetic resonance imaging (MRI) examination. This study focuses on data from the inclusion visit.
The clinical and cognitive assessments have been previously reported in the GRECogVASC study [13] and are detailed in the Supplementary Method 2. Cognitive scores were analyzed according to a validated framework [14] and provided age- and education-adjusted z scores for each domain (Supplementary Method 2). The cognitive status (subjective cognitive complaint, mild CI, dementia) was defined using the VASCOG criteria (Vascular Behavioral and Cognitive Disorders).
18F-florbetapir PET was performed according to the recommended methods [15] (Supplementary Method 3). PET images were visually evaluated by trained nuclear medicine physicians blinded to the patient clinical information. Quantitative analyses were also performed and the corresponding standardized uptake value ratios (SUVr) were calculated using the cerebellar cortex as a reference [15]. To avoid a confounding influence by the stroke lesion, the lesion was delineated on the corresponding MRI and excluded from the composite volume of interest (VOI) on the PET data. Patients with a global florbetapir SUVr ≥1.35 were considered to be amyloid positive. There was perfect agreement between the visual and quantitative interpretations (κ=1, P=0.0001). To examine whether stroke lesion could have promoted amyloid deposition, two additional analyses were performed. First, the SUVr of the peri-stroke region were determined using the Wollenweber methods (Supplementary Method 3) and compared to the homologous region in the contralateral hemisphere [10]. Second, we separately calculated the composite VOI of the ipsilesional and contralesional hemispheres. Their relationship was assessed using a correlation analysis between the composite VOIs of the ipsilesional and contralesional hemispheres.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Florbetapir PET was positive for 14 patients (mean SUVr: 1.462±0.091), corresponding to a prevalence of 15.4% (95% confidence interval: 7.97%-22.8%). The prevalence was similar (13.8%, 95% confidence interval: 6.5%-21%) in the 87 patients without prestroke CI (defined as Informant Questionnaire on Cognitive Decline in the Elderly score <55). PET imaging was delayed for a large proportion of patients due to late supplying of florbetapir, resulting in a mean poststroke time interval of approximately 2 years. This interval did not differ according to PET status (Table 1). The peristroke SUVr (1.56±0.3) was mildly lower (P=0.001) than that of the homologous VOI (1.62±0.3). The composite VOI of the ipsilesional hemisphere (1.20±0.17) was highly correlated (R=0.97, P=0.0001) with that of the contralesional hemisphere (1.21±0.17).
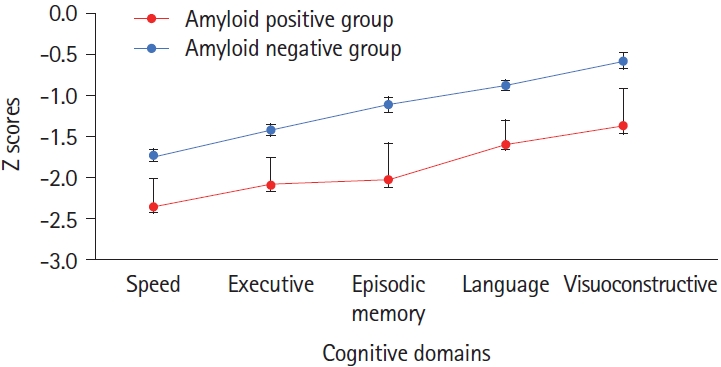
Age- and education-adjusted scores on cognitive screening tests showed greater impairment among amyloid-positive patients (Table 1). Accordingly, analysis of variance showed cognitive z scores (Figure 1) to be lower (P=0.02) for amyloid-positive patients (z: -1.886±0.289, amyloid negative patients: -1.138±0.123) and to differ according to domain (P=0.0001) due to better overall language and visuoconstructive scores, without interaction (P=0.9). The behavioral dysexecutive score did not differ (P=0.7) between groups (amyloid positive group: -1.857±2.8, amyloid negative group: -1.571±2.15).
Regarding cognitive status, logistic regression showed that dementia was more frequent (odds ratio: 6.44, 95% confidence interval: 1.14-36.6, P=0.04) in amyloid-positive (dementia: 28.6%; mild CI: 50%) than amyloid-negative patients (dementia: 7.78%; mild CI: 54.5%).
Finally, the stroke-PET time interval did not correlate with any significant findings: SUVr (R=-0.05, P=0.9), education-adjusted score on the Mini-Mental State Examination (R=0.1, P=0.3), and global cognitive score (R=-0.04, P=0.7).
The IDEA3 study shows cerebral amyloid positivity to be observed with a prevalence of 15.4% (95% confidence interval: 7.97%-22.8%) and associated with a more severe cognitive status. The present results were obtained with the largest population to date using comprehensive cognitive assessment. Together with previous reports [9-12], they support a poststroke prevalence of amyloid positivity of approximately 15%-20%. Differences between studies are likely to be due to the inclusion of patients of different ages and varying cognitive status and this should be examined in a future study. Accordingly, we found both factors to be associated with amyloid status. The present study focused on patients with at least one abnormal cognitive score at one time point, as this typically raises the question of cognitive deficit and its cause in clinical practice.
The lack of association between stroke lesion and amyloid deposit supports previous reports [10,16].
The present results demonstrate that the amyloid burden is associated with cognitive status. This supports the pioneering reports that suggested that a part of poststroke dementia is related to associated AD [6-8]. This association is independent of age (as all cognitive scores were adjusted for age and education) and poststroke time interval. The discrepancy with the three poststroke PET studies that did not find any relationship between amyloid burden and cognitive status can be explained by differing sample sizes [10-12]. Conversely, it is unlikely to be due to differences in the duration of follow-up, as negative results have been reported in long-term assessment [11,12]. We are currently assessing the effect of amyloid burden on long-term outcome, as the sole study showing a relationship with cognitive outcome documented greater cognitive decline over time [9]. The present study supports the lack of association between amyloid burden and behavioral disorders (including apathy) and depression.