Mode of Imaging Study and Endovascular Therapy for a Large Ischemic Core: Insights From the RESCUE-Japan LIMIT
Article information
Abstract
Background and Purpose
Differences in measurement of the extent of acute ischemic stroke using the Alberta Stroke Program Early Computed Tomographic Score (ASPECTS) by non-contrast computed tomography (CT-ASPECTS stratum) and diffusion-weighted imaging (DWI-ASPECTS stratum) may impact the efficacy of endovascular therapy (EVT) in patients with a large ischemic core.
Methods
The RESCUE-Japan LIMIT (Recovery by Endovascular Salvage for Cerebral Ultra-acute Embolism Japan–Large IscheMIc core Trial) was a multicenter, open-label, randomized clinical trial that evaluated the efficacy and safety of EVT in patients with ASPECTS of 3–5. CT-ASPECTS was prioritized when both CT-ASPECTS and DWI-ASPECTS were measured. The effects of EVT on the modified Rankin Scale (mRS) score at 90 days were assessed separately for each stratum.
Results
Among 183 patients, 112 (EVT group, 53; No-EVT group, 59) were in the CT-ASPECTS stratum and 71 (EVT group, 40; No-EVT group, 31) in the DWI-ASPECTS stratum. The common odds ratio (OR) (95% confidence interval) of the EVT group for one scale shift of the mRS score toward 0 was 1.29 (0.65–2.54) compared to the No-EVT group in CT-ASPECTS stratum, and 6.15 (2.46–16.3) in DWI-ASPECTS stratum with significant interaction between treatment assignment and mode of imaging study (P=0.002). There were significant interactions in the improvement of the National Institutes of Health Stroke Scale score at 48 hours (CT-ASPECTS stratum: OR, 1.95; DWIASPECTS stratum: OR, 14.5; interaction P=0.035) and mortality at 90 days (CT-ASPECTS stratum: OR, 2.07; DWI-ASPECTS stratum: OR, 0.23; interaction P=0.008).
Conclusion
Patients with ASPECTS of 3–5 on MRI benefitted more from EVT than those with ASPECTS of 3–5 on CT.
Introduction
Alberta Stroke Program Early Computed Tomographic Score (ASPECTS) on non-contrast computed tomography (NCCT) (CT-ASPECTS) is widely used to assess the extent of early ischemic changes for acute ischemic stroke [1]. ASPECTS was also applied to diffusion-weighted magnetic resonance imaging (DWI-MRI), known as DWI-ASPECTS, which had higher sensitivity than CT-ASPECTS for the detection of early ischemic changes [2]. A previous study reported a discrepancy of one point between CT-ASPECTS and DWI-ASPECTS in acute ischemic stroke [3], but it was uncertain whether such discrepancy was observed in patients with a large ischemic core.
In acute ischemic stroke with large vessel occlusion (LVO), it is important to identify patients who can benefit from endovascular therapy (EVT), especially those with a large ischemic core, because of concerns regarding potential bleeding risks of EVT [4]. Therefore, advanced imaging studies, like MRI or computed tomography perfusion (CTP), are beneficial for patient selection in EVT; however, they are not widely available [5]. In a recent study of patients in the extended time window, no significant differences were observed in the clinical outcomes of patients selected by NCCT compared with those selected by MRI or CTP [6]. However, the relationship between the mode of imaging study and the efficacy of EVT among patients with a large ischemic core was not well understood [7].
The Recovery by Endovascular Salvage for Cerebral Ultra-acute Embolism Japan–Large IscheMIc core Trial (RESCUE-Japan LIMIT) first reported that EVT was effective in patients with a large ischemic core with ASPECTS of 3–5 on either NCCT or DWI-MRI, in which 86% of enrolled patients were selected by DWI-MRI [8-10], followed by two randomized clinical trials, which confirmed better functional outcomes with EVT in the setting where NCCT was dominant [11,12]. Although DWI-ASPECTS was reported to have higher sensitivity than CT-ASPECTS [2], CT-ASPECTS is widely used for imaging study of acute ischemic stroke in many stroke centers worldwide. If the selection of imaging modalities affects the efficacy and safety of EVT in patients with a large ischemic core, these differences should be clarified. As the RESCUE-Japan LIMIT collected imaging studies of NCCT in patients primarily evaluated by DWI-MRI, we explored the differences in the efficacy of EVT in patients with a large ischemic core, which was assessed by CT-ASPECTS or DWI-ASPECTS as a pre-defined subanalysis.
Methods
Study design
This study explored whether the efficacy of EVT with medical care (EVT group) compared to medical care alone (No-EVT group) in patients with acute LVO with ASPECTS of 3–5 differed between ASPECTS measurements by NCCT (CT-ASPECTS stratum) and DWI-MRI (DWI-ASPECTS stratum). This predefined subanalysis is described in the protocol [13]. The data that support the findings of this study are available from the corresponding author upon reasonable request.
The RESCUE-Japan LIMIT was a multicenter, open-label, randomized clinical trial conducted in 45 hospitals in Japan that evaluated the efficacy and safety of EVT in patients with acute LVO with a large ischemic core. RESCUE-Japan LIMIT enrolled 203 patients from November 2018 to September 2021, with follow-up ending in December 2021 [8,13]. This trial exclusively enrolled patients with CT-ASPECTS of 3–5 or DWI-ASPECTS of 3–5. Additional eligibility criteria were: (1) acute ischemic stroke; (2) age ≥18 years; (3) National Institutes of Health Stroke Scale (NIHSS) score of 6 or higher on admission [14]; (4) modified Rankin Scale (mRS) score of 0–1 before onset [15]; (5) occlusion site at the internal carotid artery (ICA) or M1 segment of the middle cerebral artery (M1) on computed tomography angiography (CTA) or magnetic resonance angiography (MRA); (6) randomization could be completed within 6 hours from the time the patient was last known to be well, or 6–24 hours from the time the patient was last known to be well if there were no ischemic changes on fluid-attenuated inversion recovery imaging [16]; and (7) EVT could be initiated within 60 minutes from randomization. Exclusion criteria included: (1) a significant mass effect with a midline shift on NCCT or MRI, (2) evidence of acute intracranial hemorrhage (ICH) on NCCT or MRI, and (3) a high risk of hemorrhage or other conditions [13].
Ethics approval and consent to participate
This study was conducted in accordance with the principles of the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan. The protocol and consent forms were approved by the Institutional Review Boards of the Hyogo College of Medicine (approval number: 3015) and all participating hospitals. All patients or their legally authorized representatives provided written informed consent before randomization. This trial was registered at ClinicalTrials.gov (NCT03702413).
Measurements and interventions
Baseline characteristics and treatment details were obtained from neurologists at the study hospitals. The baseline characteristics included premorbid mRS score, NIHSS score, occlusion sites, ASPECTS, and laboratory tests. Stroke classification was determined at 7 (±2) days after randomization or at discharge and categorized as cardioembolic, atherothrombotic, cryptogenic, or others according to the Trial of Org 10172 in Acute Stroke Treatment classification [17].
Imaging studies were conducted using NCCT or DWI-MRI. The imaging mode was selected by the treating neurologists who were trained and certified in advance to assess ASPECTS using both NCCT and DWI-MRI [1,18]. The imaging studies were sent to a core laboratory and independently evaluated by an imaging evaluation committee [13]. The eligibility was based on the initial evaluation by the treating neurologists, but the event adjudication committee and imaging evaluation committee made the final judgments including the assessment of ASPECTS using both NCCT and DWI-MRI. In this subanalysis, we selected patients from the per-protocol population. When both NCCT and DWI-MRI were performed, the ASPECTS using NCCT was prioritized. CTA or MRA was simultaneously performed to determine the occlusion site when acute LVO was suspected on NCCT or DWI-MRI. The infarction volume on admission was retrospectively calculated on DWI-MRI or CTP using RAPID software (iSchemaView, Menlo Park, CA, USA) [19].
The EVT method was selected by the treating neurologists for patients in the EVT group. The approved devices for EVT included a stent retriever, aspiration catheter, balloon angioplasty, intracranial stent, and carotid artery stent in Japan [20]. The degree of reperfusion was measured using the Thrombolysis in the Cerebral Infarction grading system [21]. Intravenous thrombolysis (IVT) was administered to selected patients by treating neurologists. The standard dose of alteplase was 0.6 mg per kilogram of body weight according to the Japanese guidelines [22]. Other standard treatments were administered to both treatment groups based on the current guidelines [23].
Outcomes
The primary outcome of this subanalysis was an ordinal shift across the range of mRS scores toward a better outcome at 90 days (shift analysis). The secondary outcomes included an mRS score of 0–3, 0–2, and 0–1 at 90 days after onset, and an improvement of 8 points or more on the NIHSS score at 48 hours after randomization [8,13]. The safety outcomes included symptomatic ICH, defined as parenchymal hematoma type 2 (hematoma in >30% of the infarcted area with a substantial space-occupying effect) with a worsening NIHSS score by 4 points or more within 48 hours [24], any ICH within 48 hours, death (mRS score of 6) within 90 days, recurrence of ischemic stroke within 90 days, and decompressive craniectomy within 7 days after onset. The mRS score at 90 days was assessed by a neurologist or physical therapist who was blinded to the treatment assignment.
Statistical analysis
We described the characteristics of the patients according to the CT-ASPECTS and DWI-ASPECTS strata. Continuous variables are expressed as either means and standard deviations or medians and interquartile ranges, and were compared using the Student’s t-test or Wilcoxon rank-sum test based on their distributions. Categorical variables are presented as numbers and percentages, and were compared using the chi-square test or Fisher’s exact test, as appropriate.
The distributions of ordinal mRS scores were presented in the CT-ASPECTS and DWI-ASPECTS strata, and the differences in distribution between the EVT and No-EVT groups were assessed using the chi-square test. We calculated the proportion of primary and secondary outcomes in the CT-ASPECTS and DWI-ASPECTS strata and separately compared the effects of the EVT and No-EVT groups on the outcomes. We constructed an ordinal logistic regression model for the primary outcome to estimate the common odds ratios (ORs) with 95% confidence intervals (CIs) for one scale lower of the mRS score at 90 days after assessment of the proportional odds assumption. The effects of the EVT group compared with the No-EVT group on secondary outcomes are presented as ORs with 95% CIs calculated from binary logistic regression models. We constructed the same ordinal and binary logistic regression models with an interaction term between the treatment assignment and mode of imaging study to estimate the interaction P-values for the entire cohort.
All statistical analyses were performed using JMP 16.0 (SAS Institute Inc., Cary, NC, USA). Missing baseline characteristic data were not imputed and were eliminated from the corresponding analyses. All reported P-values were two-tailed, and P-values <0.05 were considered statistically significant. Because of the exploratory nature of this study, we did not adjust the P-values by considering multiplicity.
Results
Patient characteristics
Of the 203 enrolled patients, after excluding one patient who withdrew consent and 19 patients out of the per-protocol population (one patient randomized after 6 hours without MRI, two patients who had an mRS score besides 0–1 before onset, three patients who had an occlusion site besides the ICA or M1, and 13 patients who had an ASPECTS besides 3–5), 183 patients were included in this analysis (Figure 1). Among them, CT-ASPECTS was measured in 112 patients; the remaining 71 patients did not undergo NCCT. There were 88 patients who underwent both NCCT and DWI-MRI in the CT-ASPECTS stratum, and no patients with CT-ASPECTS of 3–5 and DWI-ASPECTS besides 3–5.
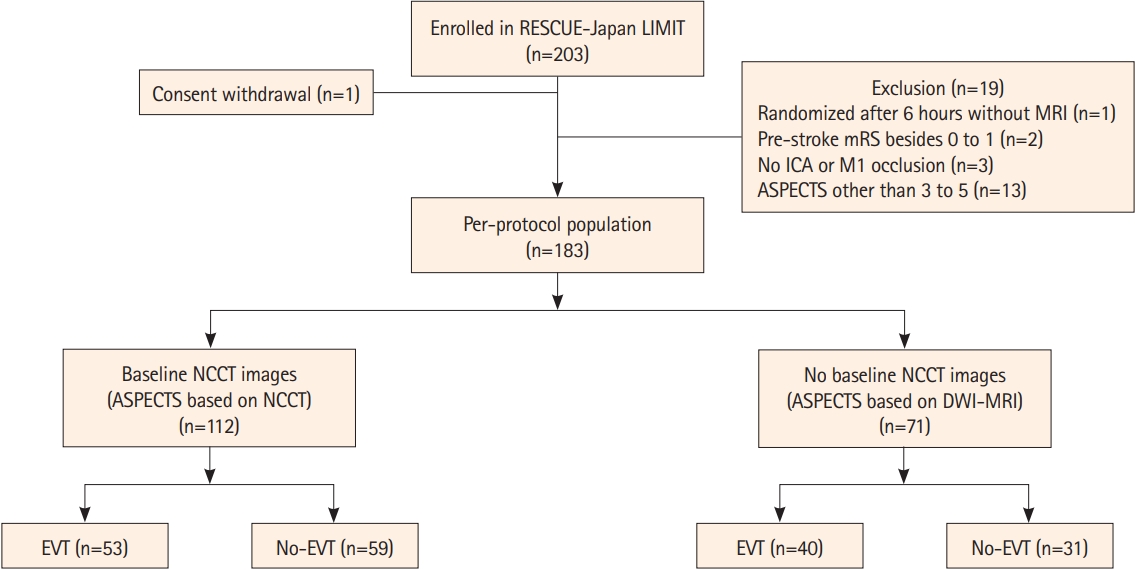
Study flowchart. RESCUE-Japan LIMIT, Recovery by Endovascular Salvage for Cerebral Ultra-acute Embolism Japan–Large IscheMIc core Trial; MRI, magnetic resonance imaging; mRS, modified Rankin Scale; ICA, internal carotid artery; M1, M1 segment of the middle cerebral artery; ASPECTS, Alberta Stroke Program Early Computed Tomographic Score; NCCT, non-contrast computed tomography; DWI-MRI, diffusion-weighted magnetic resonance imaging; EVT, endovascular therapy.
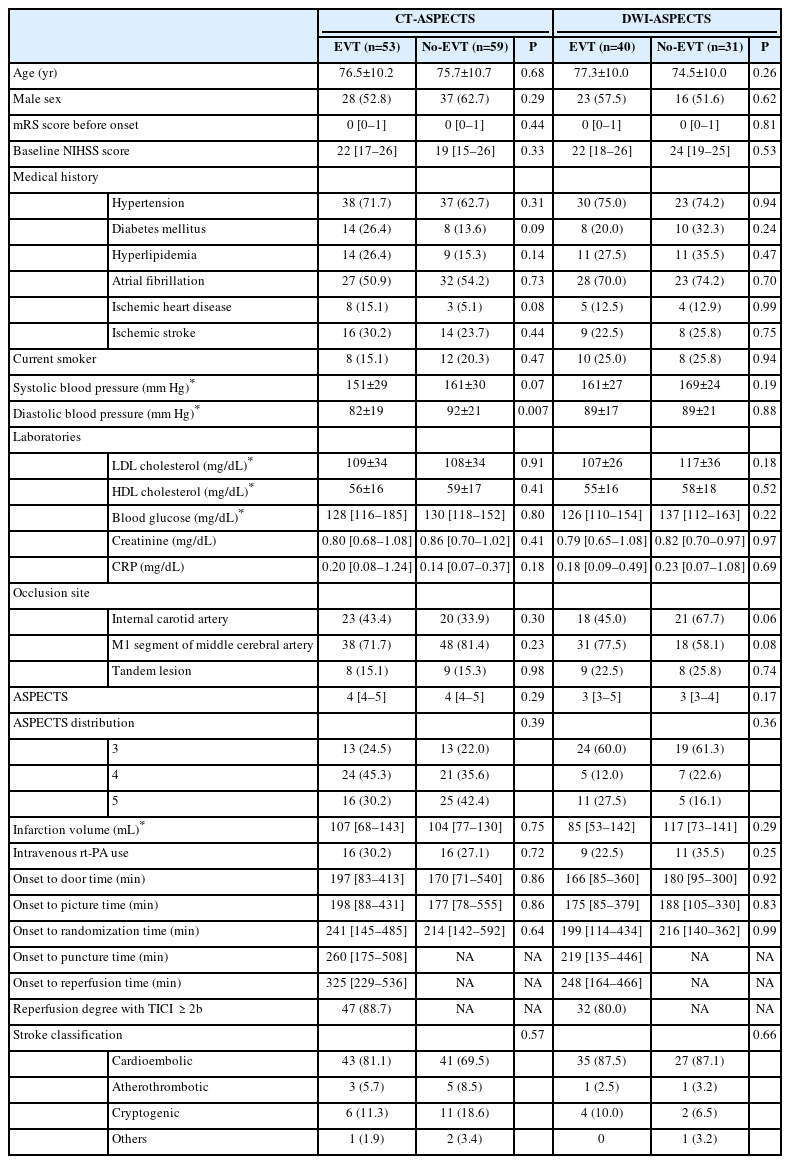
Baseline characteristics were generally similar between the CT-ASPECTS and DWI-ASPECTS strata, except for medical history of atrial fibrillation, ASPECTS, and occlusion site (Table 1). Atrial fibrillation on admission was significantly more dominant in the DWI-ASPECTS stratum, but the stroke classification was not significantly different between the strata when assessed after 7 days of admission. An ASPECTS score of 4 was dominant (40.2%) in the CT-ASPECTS stratum, while a score of 3 was dominant (60.6%) in the DWI-ASPECTS stratum. The infarction volumes on admission were comparable between the CT-ASPECTS and DWI-ASPECTS strata (Table 1). Randomization was well-maintained in both the CT-ASPECTS and DWI-ASPECTS strata (Table 2). The diastolic blood pressure in the No-EVT group was significantly higher than in the EVT group in the CT-ASPECTS stratum (Table 2); however, the other variables were well balanced.
Primary and secondary outcomes
In the CT-ASPECTS stratum, the distributions of the mRS scores at 90 days were similar between the EVT and No-EVT groups (P=0.11) (Figure 2A), whereas in the DWI-ASPECTS stratum, they significantly shifted to a lower disability in the EVT group than in the No-EVT group (P=0.004) (Figure 2B). The common OR (95% CIs) of the EVT group for one scale shift of the mRS score toward 0 was 1.29 (0.65–2.54) compared to the No-EVT group in the CT-ASPECTS stratum, while it was 6.15 (2.46–16.3) in the DWI-ASPECTS stratum with significant interaction between treatment assignment and mode of imaging study (P=0.002) (Table 3).
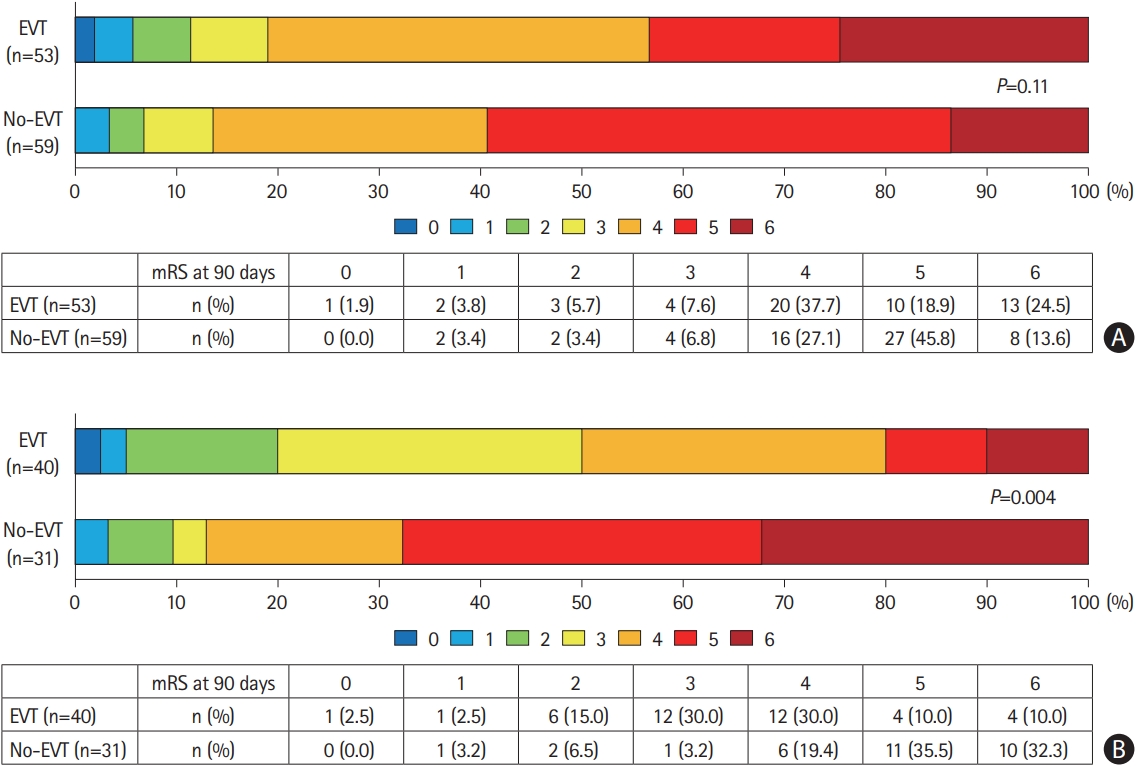
Distribution of mRS score at 90 days. (A) ASPECTS based on NCCT. (B) ASPECTS based on DWI-MRI. mRS, modified Rankin Scale; ASPECTS, Alberta Stroke Program Early Computed Tomographic Score; NCCT, non-contrast computed tomography; DWI-MRI, diffusion-weighted magnetic resonance imaging; EVT, endovascular therapy.
In the CT-ASPECTS stratum, 18.9% and 13.6% of patients in the EVT and No-EVT groups, respectively achieved an mRS score of 0–3 at 90 days; the OR (95% CI) of the EVT group compared to the No-EVT group was 1.48 (0.54–4.09), while in the DWI-ASPECTS stratum, the proportion of patients with an mRS score of 0–3 at 90 days was significantly higher in the EVT group than in the No-EVT group (50.0% vs. 12.9%; OR, 6.75; 95% CI, 1.99–22.8) without significant interaction (P=0.06). There were no significant differences in the mRS scores of 0–2 and 0–1 at 90 days between the EVT and No-EVT groups in either the CT-ASPECTS or DWI-ASPECTS strata (Table 3).
In the CT-ASPECTS stratum, the proportion of patients with an improvement of 8 points or more on the NIHSS score at 48 hours was not significantly different between the EVT and No-EVT groups (20.8% vs. 11.9%; OR, 1.95; 95% CI, 0.69–5.46), while it was higher in the EVT group than in the No-EVT group (50.0% vs. 6.5%; OR, 14.5; 95% CI, 3.04–69.1) in the DWI-ASPECTS stratum, and the interaction was significant (P=0.035) (Table 3).
The incidence of symptomatic ICH within 48 hours was small, and there was no significant difference between the EVT and No-EVT groups in both the CT-ASPECTS and DWI-ASPECTS strata. The incidence of any ICH within 48 hours was significantly higher in the EVT group than in the No-EVT group in both the CT-ASPECTS and DWI-ASPECTS strata (CT-ASPECTS stratum: OR, 2.75; 95% CI, 1.27–5.93, DWI-ASPECTS stratum: OR, 3.15; 95% CI, 1.18–8.42; interaction P=0.83) (Table 3). The beneficial effect of EVT on mortality at 90 days was not apparent in the CT-ASPECTS stratum (24.5% vs. 13.6%; OR, 2.07; 95% CI, 0.78–5.48), while EVT was significantly associated with lower mortality in the EVT group than in the No-EVT group in the DWI-ASPECTS stratum (10.0% vs. 32.3%; OR, 0.23; 95% CI, 0.06–0.84), and the interaction was statistically significant (P=0.008). Ischemic stroke recurrence within 90 days and decompressive craniectomy within 7 days were similar between the EVT and No-EVT groups in both the CT-ASPECTS and DWI-ASPECTS strata.
Discussion
This predefined subanalysis of the RESCUE-Japan LIMIT explored the relationship between the mode of imaging study and the efficacy of EVT in patients with a large ischemic core, which revealed that the efficacy of EVT differed between those who evaluated with NCCT and DWI-MRI. There were significant interactions between the mode of imaging study and the efficacy of EVT in terms of a one-scale lower mRS score at 90 days, improvement in the NIHSS score at 48 hours, and mortality at 90 days.
Several factors should be considered when interpreting these findings. Differences in imaging characteristics between NCCT and DWI-MRI are essential for evaluating the extent and time course of ischemic regions. The first step toward irreversible ischemia is hypoxia, which leads to electrical failure, followed by the disruption of Na/K-ATPase (sodium–potassium adenosine triphosphatase) activity in the cell membrane, making it visible on DWI-MRI within minutes after onset. The second step is the influx of water into the cells and the resultant cytotoxic edema, which is visible on NCCT as a loss of gray-white matter differentiation several hours after onset. The third step is disruption of the blood-brain barrier leading to vasogenic edema, which results in a more pronounced gray-white matter differentiation [25,26]. Accordingly, DWI-MRI can visualize ischemic changes earlier than NCCT, and DWI-ASPECTS was reported to be lower than CT-ASPECTS in patients with acute ischemic stroke. A previous study reported that DWI-ASPECTS was one point lower than CT-ASPECTS in patients with acute ischemic stroke with relatively smaller ischemic regions [3].
In addition, differences in the time-to-treatment intervals between the CT-ASPECTS and DWI-ASPECTS strata should be considered. Compared to the DWI-ASPECTS stratum, the median time from onset to hospital arrival in the EVT group was 30 minutes longer, and the median time from onset to puncture was 40 minutes longer in the CT-ASPECTS stratum. All patients who underwent both NCCT and DWI-MRI were categorized into the CT-ASPECTS stratum and required a longer time for imaging studies than those who underwent DWI-MRI only. In addition, some of them were transferred from hospitals where one imaging study was conducted to the hospitals participating in this study for the enrolment. As a result, the longer onset-to-reperfusion time of patients who received EVT in the CT-ASPECTS stratum might have less chance of returning to better functional outcomes [4].
Previous registry-based studies have shown similar clinical outcomes between patients with LVO evaluated by DWI-MRI and those evaluated by NCCT. A Japanese registry study reported an adjusted OR of evaluation by DWI-MRI as 1.12 for an mRS score of 0–2 at 90 days [27], while a Korean registry study reported an adjusted OR of 1.01 for the same [28], and an Austrian registry study also reported an adjusted OR of 0.87 [29]. In a recent cohort study of patients with an extended time window for EVT, there were no significant differences in the clinical outcomes of patients selected by NCCT compared with those selected by MRI or CTP [6]. Our subanalysis of the randomized clinical trial suggested the efficacy of EVT was superior in patients evaluated by DWI-MRI than in those evaluated by NCCT. Considering the potential delay in EVT in the CT-ASPECTS stratum, future research is needed to elucidate the relationship between the mode of imaging and the efficacy of EVT in patients with a large ischemic core.
The imaging study for the potential candidates of EVT for acute LVO should provide accurate diagnosis of how much irreversibly injured brain tissue is present, and this requirement is especially crucial for patients with a large ischemic core because of concern for higher bleeding risks of EVT in such patients [4]. Our previous subanalysis of the RESCUE-Japan LIMIT indicated that EVT was not associated with improved functional outcomes at 90 days and was associated with higher incidence of symptomatic ICH in patients with ASPECTS of 3 or less [30]. Collaterally, retrospective cohort studies also reported that DWI-ASPECTS of 3 or less reliably predicted those with infarction volumes more than 100 mL [31], and a 100-mL infarction volumes was the discrimination point of poor functional outcome [32]. In fact, a previous cohort study which observed patients with acute LVO who were evaluated with both DWI-ASPECTS and CT-ASPECTS reported that DWI-ASPECTS offered better prediction of a large ischemic core and functional outcomes than CT-ASPECTS [33]. Our findings were consistent to these reports and explicated them to those with a large ischemic core. Furthermore, a previous registry study showed that both asymptomatic and symptomatic ICH after EVT in patients with acute LVO were associated with worse functional outcomes [34]. The meta-analysis from HERMES (the Highly Effective Reperfusion evaluated in Multiple Endovascular Stroke Trials) showed a higher rate of symptomatic ICH after EVT in patients with low ASPECTS, but it did not report the association between symptomatic ICH and functional outcomes [35]. MRI with susceptibility-weighted imaging or T2* weighted imaging could provide more accurate qualitative information of cerebral microbleeds, which were reported to increase ICH after EVT [36]. However, the need to withhold EVT in patients with a large ischemic core who were detected to have such cerebral microbleeds should be investigated. Therefore, the selection of imaging modalities is another important factor in EVT for acute LVO with a large ischemic core.
Our study has several limitations. First, the treating neurologists selected imaging studies to assess ASPECTS using NCCT, DWI-MRI, or both. The selection of imaging studies was affected by several factors such as patient conditions, participating hospitals, and admission status (direct admission or transfer from primary stroke centers). Therefore, such selection bias might have affected the differences in the cohorts between the CT-ASPECTS and DWI-ASPECTS strata, although random allocation of EVT provided fair comparisons within the cohort. Second, sample size estimation was not performed for the subgroup analyses in this randomized clinical trial. Therefore, the power to detect clinically relevant findings within the CT-ASPECTS and DWI-ASPECTS strata was not sufficiently large. Finally, this study was conducted in Japan, where IVT is less utilized and other management strategies, such as rehabilitation or medication use, are different from those in Western countries. Therefore, the effects of differences in imaging studies on functional outcomes in patients with acute LVO and a large ischemic core should be evaluated in other settings.
Conclusions
The efficacy and safety of EVT in patients with acute LVO with an ASPECTS score of 3–5 differed between those evaluated using NCCT and DWI-MRI. In addition to the advancement of EVT, the selection of imaging modalities should be supplementary aspects to improve functional outcomes in patients with a low ASPECTS. Future large-scale observations should provide reliable information regarding this issue.
Notes
Funding statement
This study was supported in part by the Mihara Cerebrovascular Disorder Research Promotion Fund and the Japanese Society for Neuroendovascular Therapy (JSNET). The funding sources did not participate in any part of the study from conception to article preparation.
Conflicts of interest
Dr. Sakakibara reports a manuscript fee from Medicus Shuppan. Dr. Uchida reports lecturer fees from Daiichi Sankyo, Bristol-Myers Squibb, Stryker, and Medtronic. Dr. Yoshimura reports research grants from Stryker, Siemens Healthineers, Bristol-Myers Squibb, Sanofi, Eisai, Daiichi Sankyo, Teijin Pharma, Chugai Pharmaceutical, HEALIOS, Asahi Kasei Medical, Kowa, and CSL Behring, and lecturer fees from Stryker, Medtronic, Johnson & Johnson, Kaneka, Terumo, Biomedical Solutions, Boehringer-Ingelheim, Daiichi Sankyo, Bayer, and Bristol-Meyers Squibb. Dr. Sakai reports research grants from Biomedical Solutions, Medtronic, and Terumo; lecturer fees from Asahi-Intec, Biomedical Solutions, Medtronic, and Terumo; and membership in the advisory boards of Johnson & Johnson, Medtronic, and Terumo. Dr. Yamagami reports a research grant from Bristol-Myers Squibb; lecturer fees from Stryker, Medtronic, Terumo, Johnson & Johnson, Biomedical Solutions, and Medico’s Hirata; and membership of the advisory board for Daiichi Sankyo. Dr. Toyoda reports lecturer fees from Otsuka, Novartis, Bayer, Daiichi Sankyo, Bristol Myers Squibb, and Abbott Medical. Dr. Matsumaru reports lecturer fees from Medtronic, Stryker, Terumo, Johnson & Johnson, Kaneka, and Jimro. Dr. Matsumoto reports lecturer fees from Kaneka, Medico’s Hirata, Fuji Systems, GE Healthcare, Otsuka, Takeda, Century Medical, Terumo, Medtronic, and Stryker. Dr. Kimura reports research grants from CSL Behring, EP-CRSU, Amgen Astellas BioPharma, Alexion, Eisai, Kyowa Kirin, Daiichi Sankyo, Teijin, Medtronic, Bristol-Myers Squibb, Bayer, Boehringer-Ingelheim and Helios; and lecturer’s fees from Daiichi Sankyo, Boehringer Ingelheim, Bristol-Myers Squibb, Bayer, Takeda, Medtronic, Otsuka, FP, Alexion, Nippon, Chugai, Kyowa Kirin, Abbott, Shire PLC, Sanofi, CSL Behring, Novartis, Toa Eiyo, Medico’s Hirata and Helios. Dr. Inoue reports lecturer fees from Bayer, Bristol-Myers Squibb, and Medico’s Hirata, and manuscript fees from Gakken and Hokuryukan. Dr. Tanaka reports lecturer fees from Johnson & Johnson and Stryker. Dr. Yoshimoto reports lecturer fees from Takeda Pharmaceuticals and Nippon Boehringer Ingelheim. Dr. Koge reports lecturer fees from Medtronic. Dr. Beppu reports a manuscript fee from Medicus Shuppan. Dr. Shirakawa reports lecturer fees from Stryker, Terumo, Johnson & Johnson, and Medtronic. Dr. Morimoto reports lecturer fees from AstraZeneca, Bristol-Myers Squibb, Daiichi Sankyo, Japan Lifeline, Kowa, Pfizer, and Tsumura; manuscript fees from Bristol-Myers Squibb and Pfizer; and the Advisory Boards for Novartis and Teijin. Drs. Ishikura, Ando, and Yoshida declare no conflicts of interest.
Author contribution
Conceptualization: FS, KU, SY, NS, HY, TM. Study design: FS, KU, SY, NS, HY, KT (Kazunori Toyoda), YM (Yuji Matsumaru), YM (Matsumoto Yasushi), KK, TM. Methodology: FS, KU, RI, MI, KA, AY, KT (Kanta Tanaka), TY, JK, TM. Data collection: FS, KU, SY, HY, YM (Yuji Matsumaru), YM (Yasushi Matsumoto), KK, MB, MS. Investigation: FS, KU, SY, NS, HY, KT (Kazunori Toyoda), YM (Yuji Matsumaru), YM (Matsumoto Yasushi), KK, RI, MI, KA, AY, KT (Kanta Tanaka), TY, JK, MB, MS, TM. Statistical analysis: FS, KU, TM. Writing—original draft: FS, KU, TM. Writing—review & editing: SY, NS, HY, KT (Kazunori Toyota), YM (Yuji Matsumaru), YM (Matsumoto Yasushi), KK, RI, MI, KA, AY, KT (Kanta Tanaka), TY, JK, MB, MS. Funding acquisition: SY. Approval of final manuscript: all authors.
Acknowledgements
We thank all the investigators for their efforts in conducting the RESCUE-Japan LIMIT.