Cerebral Arterial Calcification Is an Imaging Prognostic Marker for Revascularization Treatment of Acute Middle Cerebral Arterial Occlusion
Article information
Abstract
Background and Purpose
To study the significance of intracranial artery calcification as a prognostic marker for acute ischemic stroke patients undergoing revascularization treatment after middle cerebral artery (MCA) trunk occlusion.
Methods
Patients with acute MCA trunk occlusion, who underwent intravenous and/or intra-arterial revascularization treatment, were enrolled. Intracranial artery calcification scores were calculated by counting calcified intracranial arteries among major seven arteries on computed tomographic angiography. Patients were divided into high (HCB; score ≥3) or low calcification burden (LCB; score <3) groups. Demographic, imaging, and outcome data were compared, and whether HCB is a prognostic factor was evaluated. Grave prognosis was defined as modified Rankin Scale 5-6 for this study.
Results
Of 80 enrolled patients, the HCB group comprised 15 patients, who were older, and more commonly had diabetes than patients in the LCB group. Initial National Institutes of Health Stroke Scale (NIHSS) scores did not differ (HCB 13.3±2.7 vs. LCB 14.6±3.8) between groups. The final good reperfusion after revascularization treatment (thrombolysis in cerebral infarction score 2b-3, HCB 66.7% vs. LCB 69.2%) was similarly achieved in both groups. However, the HCB group had significantly higher NIHSS scores at discharge (16.0±12.3 vs. 7.9±8.3), and more frequent grave outcome at 3 months (57.1% vs. 22.0%) than the LCB group. HCB was proven as an independent predictor for grave outcome at 3 months when several confounding factors were adjusted (odds ratio 4.135, 95% confidence interval, 1.045-16.359, P=0.043).
Conclusions
Intracranial HCB was associated with grave prognosis in patients who have undergone revascularization for acute MCA trunk occlusion.
Introduction
Intracranial calcified arteries are encountered in approximately 85% of patients with acute ischemic stroke.1 Intra-arterial calcification is usually accompanied by coronary atherosclerosis, and is frequently observed in the intracranial arteries.2,3 Recent studies have indicated that intracranial arterial calcification (IAC) is associated with a poor outcome in acute ischemic stroke.4,5 In the case of coronary disease, high coronary artery calcification scores can predict future ischemic coronary syndromes.6 In patients with acute coronary syndromes that received urgent intervention, moderate to severe calcification was significantly associated with higher mortality rates 1 year after the intervention.7
The higher mortality rates are thought to be associated with complexity of coronary interventions, procedure related complications, and major adverse cardiac events such as target lesion revascularization, and non-target lesion revascularization.8,9
The significance of IAC in ischemic stroke, however, has not yet been determined. Additionally, the clinical significance of IAC in acute stroke patients who had cerebral arterial occlusion and received revascularization treatment remains unclear, for previous studies had only concluded that associations between cerebral artery calcification and mortality or poor functional outcome in the first year after ischemic stroke are mainly age and stroke severity driven.5
Revascularization after acute ischemic stroke is a developing therapy.10 Intravenous (IV) recombinant tissue plasminogen activator (rtPA) was the first agent approved for treating acute ischemic stroke.11 Recently, intra-arterial (IA) revascularization therapies, including fibrinolysis and mechanical thrombectomy,12,13,14,15,16,17,18,19,20 have been used on the grounds that early recanalization guarantees a better prognosis.21 However, approximately 40% of these patients fail to show neurological recovery, even with successful revascularization.22,23 Therefore, a reliable predictor for successful revascularization therapy would be clinically valuable.
The aim of the present study was to examine the clinical significance of a high IAC burden, and to clarify the factors that are associated with calcification burdens that may influence the outcomes in these patients. To achieve this goal, we used computerized tomography (CT) angiography in patients with acute middle cerebral artery (MCA) trunk occlusion who underwent hyperacute revascularization therapy.
Methods
Study population
We performed a retrospective study based on consecutive patients enrolled to a hospital stroke database that was approved by the Institutional Review Board and performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments. We registered in our thrombolysis database 361 patients with acute ischemic stroke who underwent revascularization therapy between January 2006 and September 2011. From these 361 patients, we selected patients who met the following inclusion criteria: (1) acute stroke due to an occlusion in the main trunk of the MCA identified on initial CT angiography performed within 6 h of onset, (2) National Institutes of Health Stroke Scale (NIHSS) score ≥5, (3) IV and/or IA treatment, and (4) transfemoral cerebral angiography performed during the hyperacute period. The first and fourth criteria were included to evaluate leptomeningeal collaterals and final reperfusion status, respectively. If a frank hypodensity was seen in more than one third of MCA territory, the patient did not undertake IA treatment.
Protocols
CT scans (including non-enhanced [NECT], postcontrast axial parenchymal images, and CT angiography; SOMATOM Sensation 16, Siemens, Erlangen, Germany) were obtained for each patient after they were admitted to the emergency department. Noncontrast and postcontrast CT scans were performed by use of the axial technique with 120 kVp, 270 mA, and 5-mm section thickness reconstructions. CTA was performed by scanning from the vertex to aortic arch by use of the following parameters: 0.5 s/rotation, pitch, 1.3; collimation, 0.75 mm; maximal mA, 170; kVp, 100; field of view, 22 cm. Nonionic contrast material (80-100 mL) was administered by power injector at 4-5 mL/s into an antecubital vein with a semi-automatic contrast bolus triggering technique. The CTA source images were postprocessed to create coronal, sagittal, and axial multiplanar reformats in maximum intensity projection (MIP) images and volume rendered 3D images. IV rtPA was administered as soon as the infusion could be started, which typically was within 3 hours of symptom onset. Patients without contraindications for endovascular treatment were sent to the angiographic suite. If the initial angiography showed occlusion, they were treated immediately using the IA approach when the onset-to-groin puncture interval was expected to be less than 6 hours, irrespective of IV rtPA infusion. Endovascular revascularization treatment started based on transfemoral cerebral angiography (Allura Xper FD20/10, Philips Healthcare, Best, the Netherlands). The endovascular treatment methods were determined by interventionists' discretion.
NECT was performed after the endovascular procedure. Magnetic resonance imaging (MRI; Achieva, Philips Healthcare, Best, the Netherlands), including diffusion-weighted imaging, was performed within 2 days of symptom onset for patients enrolled in the study before January 1, 2010, but was performed after IV rtPA infusion and before IA procedures for patients enrolled after this date. NECT, CT angiography, and MRI were repeated for all patients within 7 days of revascularization therapy. Neurological scores, including the NIHSS and the modified Rankin Scale (mRS), were determined daily until discharge, and then mRS was rated at the 3rd month following their discharge. If the patient could not come to the clinic, mRS was measured by phone interviews. Well-trained nurses determined the neurological scores in outpatient clinics at follow up. In the present study, an mRS score of 5-6 was considered as a grave outcome.
Clinical and imaging data
Patient demographics, vascular risk factors, stroke etiology and laboratory results, and critical pathway data were retrieved from our Acute Ischemic Stroke and Thrombolysis databases. We further evaluated the first NECT scan and CT angiography obtained for each patient using the Alberta Stroke Program Early CT (ASPECTS) and IAC scores, respectively. The ASPECTS was determined from the initial NECT; scores ≥8 were considered high and those <8 were considered low.24 IAC scores were determined from CT angiography MIP images using a previously described method in which the number of intracranial calcified arteries was counted.1 Calcification was defined as the presence of hyperdense foci along the artery with a peak intensity >130 Hounsfield units (HU) with adjustments of window setting to differentiate contrast.1,25 This semi-quantitative scoring system was applied to 7 intracranial arteries: the right and left internal carotid starting from the cavernous segment to the communicating segment, the right and left middle cerebral, the right and left vertebral, and the basilar arteries. The IAC score was the number of arteries showing calcification, and therefore ranged from 0 (no calcification) to 7 (calcification in all 7 intracranial arteries examined). Patients were assigned to 1 of 2 groups depending on their IAC score. Patients with low IAC scores (<3) were assigned to the low calcification burden (LCB) group, whereas patients with high IAC scores (≥3) were assigned to the high calcification burden (HCB) group (Figure 1). From our preliminary receiver operating characteristic curve analysis, dichotomizing 0-2 and 3-7 had the largest area under the receiver operating characteristic curve among various combinations for grave outcome. On this ground, patients with low IAC scores (<3) were assigned to the low calcification burden (LCB) group, whereas patients with high IAC scores (≥3) were assigned to the high calcification burden (HCB) group (Figure 1). Before IA treatment began, leptomeningeal collaterals from the ipsilateral anterior cerebral artery were evaluated and graded with transfemoral cerebral angiography, as previously reported.26 This collateral grade was not evaluated when the MCA was fully or partially recanalized. Following either IV or IA treatment, final reperfusion status was evaluated using the thrombolysis in cerebral infarction (TICI) system.26 A TICI score of 2b-3 (reperfusion in ≥67%) was considered to indicate a good reperfusion, whereas lower TICI scores indicated a poor reperfusion.27 Post-treatment intracerebral hemorrhages were evaluated according to criteria defined by the European Cooperative Acute Stroke Study.28 The final infarct volume was determined using the total volume of diffusion restriction lesions observed on MRIs obtained between 1 and 7 days following revascularization treatment. When MRI was unavailable, the final infarct volume was determined from the total hypodensity observed on CTs obtained during the same time period. The infarct volume was calculated using a previously described semi-quantitative method.29 Calcification scoring and other imaging analyses were performed with consensus by raters who were blinded to the clinical information (S. J. Lee, J. W. Choi, J. S. Lee).

Representative patient categorization based on the number of intracranial arterial calcifications. One calcification is shown in the terminal segment of the right internal carotid artery. This case was assigned to the low calcification burden group. Five calcifications are shown in the right middle cerebral artery, the right and left terminal internal carotid, and the distal vertebral arteries. This case was assigned to the high calcification burden group.
Statistical analysis
Differences between the LCB and HCB groups were analyzed using a χ2 test for categorical variables and Student's t-test for continuous variables. Multiple logistic regression analysis was performed to verify the significance of IAC burden as a risk factor for grave outcomes. A P value of <0.05 was considered statistically significant. All statistical analyses were performed with statistical software IBM SPSS Statistics 19 (Chicago, IL, USA).
Results
Demographic and clinical characteristics
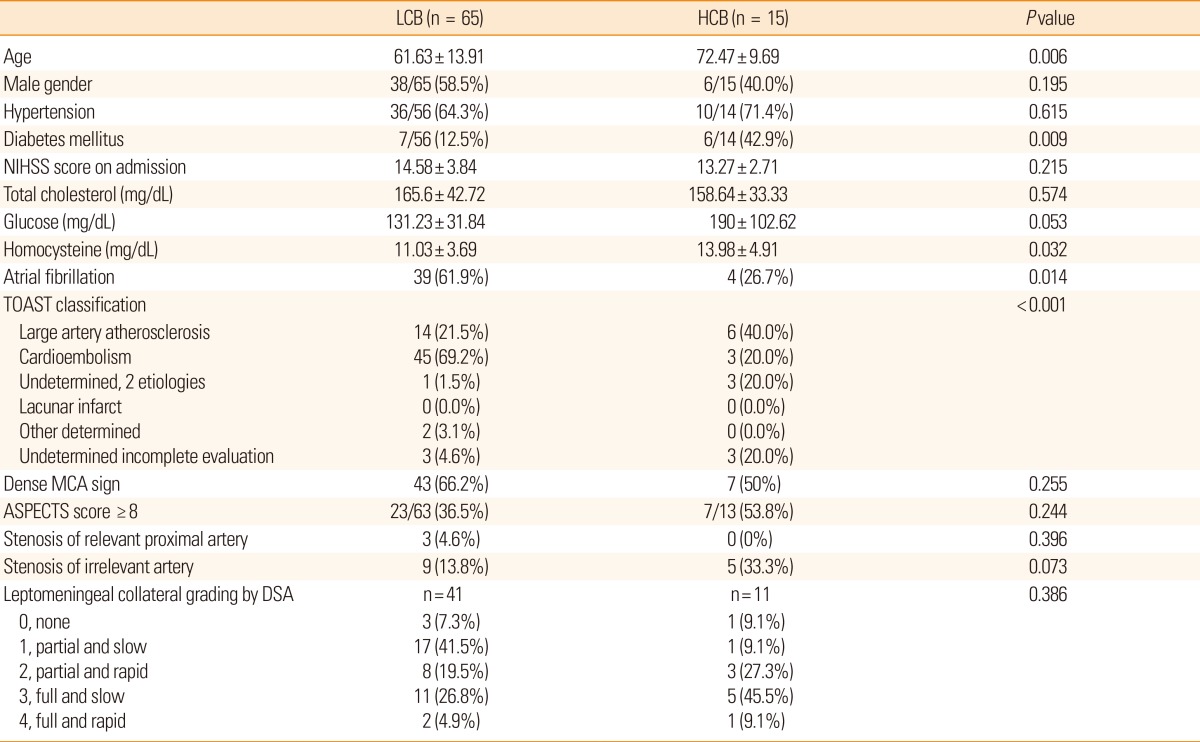
The baseline characteristics of patients in both groups are presented in Table 1. Of the 80 patients studied, 65 were assigned to the LCB group and 15 were assigned to the HCB group. Patients in the HCB group were significantly older (72.5±9.7 years vs. 61.6±13.9 years, P=0.006), and were more likely to have diabetes (42.9% vs. 12.5%, P=0.009) than those in the LCB group. Serum homocysteine levels also were significantly higher in the HCB group (14.0±4.9 µmol/L vs. 11.0±3.7 µmol/L, P=0.032) than in the LCB group. The HCB group included a higher proportion of large artery atherosclerosis, whereas the LCB group included a higher proportion of cardioembolic stroke (P<0.001).
Our analysis of the initial CT and transfemoral cerebral angiographic findings failed to reveal significant differences between groups in the number of patients showing a dense MCA sign, ASPECTS scores ≥8, or the extent of leptomeningeal collaterals from the ipsilateral anterior cerebral artery.
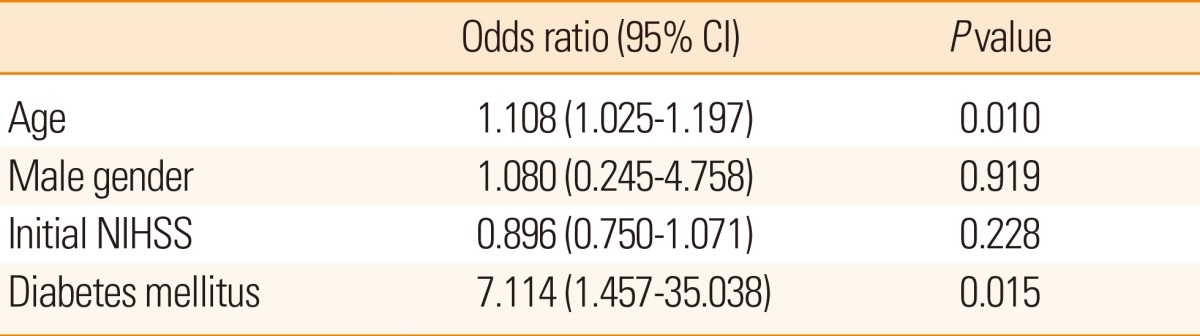
A multiple regression model showed that both age and presence of diabetes mellitus were independent predictors for high IAC score (Table 2).
Therapeutic regimens and imaging outcomes
There were no differences in treatment regimens or imaging outcomes between the 2 groups (Table 3). IV rtPA was infused in 12 (80%) patients in the HCB group, and 46 (70.8%) patients in the LCB group (P=0.470). The IA recanalization procedure was performed in 12 (80.0%) patients in the HCB group and in 55 (84.6%) patients in the LCB group (P=0.255). There was no significant difference in the frequency of each IA method. IV tPA infusion time or procedure times did not differ between groups. The final angiographic outcome, as assessed by the proportion of patients with good reperfusion, did not differ between groups. The final infarct volumes (HCB: 144.1±115.6 mL vs. LCB: 120.8±129.0 mL, P=0.523) did not differ between groups.
Poor outcomes
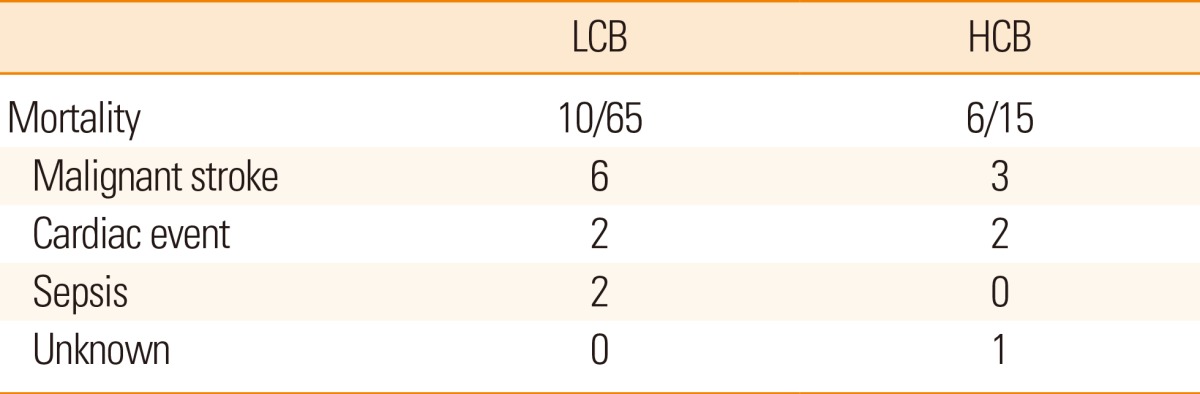
Finally, the HCB group showed poorer outcomes compared to the LCB group (Table 3). The NIHSS score at discharge was significantly higher in the HCB group than in the LCB group (16.0±12.3 vs. 7.9±8.3, P=0.003). Patients in the HCB group more frequently showed deterioration (>3 point increases) in NIHSS scores than patients in the LCB group (40.0% vs. 6.5%, P=0.001). Moreover, a grave outcome (mRS 5-6) at 3 months after stroke onset was more common in the HCB group than in the LCB group (57.1% vs. 22.0%, P=0.009). The causes of death were similar between the groups (Table 4). In a multiple logistic regression model, HCB was an independent predictor for a poor outcome at 3 months after adjusting for age, sex, initial NIHSS score, and the reperfusion grade (odds ratio 4.135, 95% confidence interval, 1.045-16.359, P=0.043; Table 5). When presence of diabetes mellitus and atrial fibrillation were incorporated into those confounding factors, HCB was not an independent predictor. Diabetes mellitus appeared to be stronger predictor for clinical outcome.
Discussion
In the present study, we demonstrate that IAC significantly influences poor outcomes in a homogenous population of patients with acute ischemic stroke. Specifically, patients with MCA trunk occlusion who underwent revascularization therapy and with an HCB in their intracranial arteries rarely showed neurological improvement. In general, cardioembolic stroke results in greater disability than does atherosclerotic stroke. In the present study, however, the LCB group, which consisted mostly of cardioembolism, showed less disability than the HCB group, which consisted mostly of atherosclerosis.
A poorer disability in patients with HCB was attributed to neurological deterioration following revascularization treatments. One may presume that intracranial HCB is associated with coronary artery disease, and that cardiac disease may influence mortality and morbidity following stroke. However, there was no difference in cardiac events between groups when mortality occurred within 3 months. Most HCB patients that demonstrated grave outcome, which was defined as being bed-ridden or deceased, often showed deterioration and little improvement in their neurological status (NIHSS scores). A previous study of acute ischemic stroke patients who underwent revascularization therapy revealed that approximately 60% of the patients showed neurological improvement, whereas the remaining 40% showed no improvement.22 As for our patients, approximately 80% of the LCB patients showed improvement in their neurological status during their hospital admission. This outcome can be considered good when compared to those described in the previous report.22 In our study, however, the majority of patients with HCB showed no neurological recovery and often showed further deterioration.
Even though the post-treatment reperfusion grade was as high as 70%, which immediate revascularization usually predicts a good outcome,30 HCB patients generally showed poor neurological recovery. This poor neurological recovery could be explained by vascular aging, a phenomenon associated with arterial calcification. In addition to chronological aging31 and accumulation of advanced glycation end products,32 calcium deposition is a primary component of vascular aging, which is characterized by endothelial dysfunction.32 A number of previous reports support such an interpretation. Firstly, vascular aging may be associated with post-ischemic expansion of the infarction due to poor recovery from the ischemia-reperfusion injury.33 Secondly, chronically high levels of reactive oxygen species accompanying the vascular calcification process can hamper neovascularization after reperfusion.34 High levels of reactive oxygen species significantly impair endothelial progenitor cell function, which further increases reactive oxygen species levels. Many diseases such as diabetes type I and II are associated with a decrease in both the function and number of endothelial progenitor cells.35 Endothelial progenitor cells are essential to neovascularization in post-stroke repair; therefore, any disturbance in their activity could impair neurological recovery.36,37
Intracranial HCB can be an imaging marker for predicting a poorer prognosis. In the present study, the therapeutic regimens and radiological outcomes did not differ between the HCB and LCB groups. However, some well-known vascular factors associated with arterial calcification differed between the groups, such as age, co-morbidity with diabetes mellitus, and initial serum levels of glucose and homocysteine.38,39,40 Among these factors, an older age and diabetes mellitus are regarded as prognostic factors for a poorer outcome, and European guidelines limit intravenous rtPA infusion in acute stroke patients who are over 80 years of age, or who have diabetes with a history of stroke.41,42 Nevertheless, available data for factors such as an older age and the presence of diabetes mellitus are inconclusive.27,43,44,45,46 Our results show that intracranial HCB was associated with both age and diabetes and is more likely to indicate a poor outcome because this measure appears to reflect such major risk factors, none of which, however, can directly predict the clinical outcome. IAC burden can be easily measured even with NECT,47 which is typically performed prior to thrombolytic treatment. Further studies that include large clinical trials for stroke thrombolytic treatment should prove useful in confirming intracranial HCB as a prognostic marker.
There are several limitations to our study. Firstly, instead of using a quantitative calcification burden score that was used previously to assess coronary atherosclerosis, we used a semi-quantitative scoring system. We chose this method of analysis because quantitative analysis of IAC burden is not easily performed due to the close proximity of bony structures. Secondly, this study examined patients with acute MCA trunk occlusion, and therefore might not reflect the calcification burdens for patients treated with revascularization therapy for other vascular structures. Our strict occlusion site criterion was selected to allow a homogenous assessment of collateral grading that primarily measures the ipsilateral anterior cerebral artery leptomeningeal collaterals, which cannot be readily applied to distal internal carotid or basilar artery occlusions. Further studies are required to validate the significance of calcification burdens on outcomes with other vascular beds.
Conclusions
Intracranial HCB was associated with a grave prognosis after revascularization treatment for hyperacute ischemic stroke. In our analysis, we focused on patients who had an MCA trunk occlusion. Thus, further studies examining other forms of intracranial arterial occlusion are required to establish HCB as a reliable marker.
Notes
This work was supported by the new faculty research fund of the Ajou University School of Medicine.
The authors have no financial conflicts of interest.