Introduction
Cerebral small vessel disease (SVD) has been recognized since the 19th century, when it was diagnosed at autopsy and correlated with clinical features. However, studies were at that time few, and the field did not make any major advance until the work of C Miller Fisher in the 1960-ies and onwards. Fisher described the classical lacunar syndromes and the vascular pathology underlying lacunes.1,2 The introduction of computerized tomography in the 1970-ies prompted a phase of clinical-CT correlations, and studies on risk factors and prognosis of lacunar infarcts. However, the introduction of magnetic resonance imaging (MRI) has revised many of these correlations and features of lacunar infarcts. MRI also revolutionized the study of silent cerebral infarcts and delineated their correlation with risk factors and prognostic implications on vascular risk and cognition.3 MRI has also been critical in delineating other features of the spectrum of cerebral small vessel disease such as white matter hyperintensities (white matter disease), cerebral microbleeds, cerebral atrophy and cerebral microinfarcts.
Imaging has played a crucial role in studying cerebral small vessel disease, and its role will likely increase further in the future as more precise techniques appear. By definition, cerebral small vessel disease is "small," hence spatial resolution is a critical element in its study. This review will outline recent developments in SVD concepts as they relate to advanced brain imaging.
Symptomatic lacunar infarcts: clinic-anatomical definitions revised by imaging
Lacunar infarcts account for up to about a quarter of all ischemic strokes, constitute one of the classical stroke subtypes, and are included as a separate entity in all major ischemic stroke classification algorithms. CT was used in the early studies that tested the diagnostic validity of subtyping ischemic stroke into the lacunar infarct subtype. In most studies patients with a visualized small deep infarct were usually lumped together with patients with no imaging abnormality. Most studies claimed that the clinical lacunar syndromes were quite highly suggestive of a small vessel disease as the likely underlying cause. However, the research field entered a new era with the advent of MRI, and in particular diffusion weighted-MRI, which highly reliably can identify acute ischemic abnormalities and separate acute from old lesions. Two early small studies showed that diffusion weighted-MRI (DW-MRI) visualized multiple regions of ischemia not compatible with lacunar infarcts in 16%4 and 29%.5 Wessels et al.6 reported the finding of a single subcortical ischemic area in only 59% of patients, whereas 22% had large or scattered lesions in one territory and 19% had multiple lesions in multiple territories. Potter et al.7 studied associations of clinical stroke misclassifications ("clinical-imaging dissociation") in 137 patients with an acute ischemic stroke presenting with a mild cortical or lacunar syndrome; 21/93 (23%) patients with a cortical syndrome had an acute lacunar infarct, whereas 7/44 (16%) patients with a lacunar syndrome had an acute cortical infarct. Another study by Asdaghi et al.8 compared the lacunar syndrome subtype of the Oxfordshire Community Stroke Project classification with DW-MRI findings, and reported a positive predictive value for the LACI subtype of only 40%-60%.
These findings clearly indicate that MRI is the required imaging modality in investigations for symptomatic cerebral small vessel disease in clinical practice as well as in research if the diagnosis should be made with adequate precision. However, it should be recognized that even MRI fails to visualize a proportion of acute small subcortical infarcts, presumably because of their small size.9
Imaging evolution of symptomatic lacunar infarcts: not always a "lacune"
Traditionally, the upper size limit of lacunar infarcts were suggested to be 15 mm as measured from CT findings. MRI studies have shown that the size on DW-MRI in the acute phase can be larger than 15 mm, and possibly up to 20 mm on axial sections, and that a strict size criterion is not valid.10 Lesions in the basal ganglia and internal capsule that are larger than 20 mm and seem to be due to simultaneous infarction in several penetrating arteries should not be labeled small subcortical (or lacunar) infarcts, but rather striatocapsular infarcts, and infarct subtype with a different cause.11 Acute lacunar infarcts diminish in size with time, and on late images the diameter is less than 15 mm.
The term "lacune" has been used in many different contexts, potentially creating confusion. In one of his late papers, C. Miller Fisher wrote: "Historically, the original small vessel disease feature was the lacune (hole), which derived from French for a small fluid-filled cavity that was thought to mark the healed stage of a small deep brain infarct. The term was adopted into English. By a process of medico-linguistic evolution, the precavitary phase became the lacunar infarct, the associated clinical entity became the lacunar stroke and the neurological features became the lacunar syndrome."12 Thus, the term lacune is a neuropathological term that refers to a finding of a cavitation. On MRI lacune is defined from FLAIR images showing a central CSF-like hypointensity with a surrounding rim of hyperintensity.
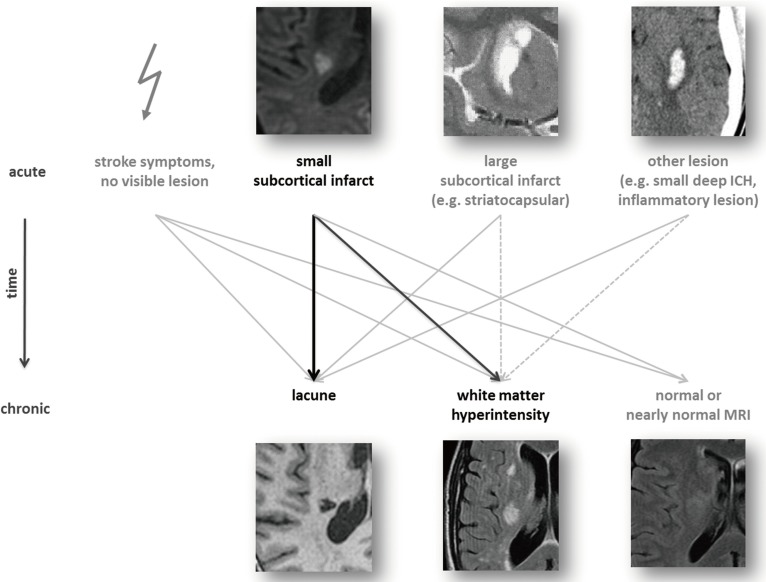
Follow-up studies of patients with acute lacunar infarcts visualized in the acute phase by MRI, have showed that not all such infarcts cavitate with time and appear as "lacunes" on later imaging studies. A proportion of acute infarcts later appear as white matter hyperintensities, whereas others are not visualized at all with time. Furthermore, also larger subcortical infarcts (striatocapsular infarcts) and other lesions (e. g., a small deep intracebral hemorrhage or an inflammatory lesion) may give the late imaging appearance of a cavitated lacune or white matter hyperintensity.13 As a consequence, it needs to be recognized that the distinction between previous symptomatic cerebral infarcts, silent brain infarcts and white matter hyperintensities of presumed vascular origin is less sharp than previously recognized, or reported in scientific studies, as interpreted from late neuroimaging findings alone (Figure 1). Two studies have reported proportions of 28% and 94% of acute small deep infarcts that cavitate.14,15 Cavitation may be related to time and size of the lesion, but also to neuroimaging methods such as MRI sequences.16 Further studies on the sequential imaging appearance of small acute lacunar infarcts are warrented.
Imaging of intracranial atherosclerosis and small penetrating artery disease causing acute lacunar infarcts
Imaging in vivo of small penetrating arteries have earlier been elusive, and only in rare instances have a presumed thrombus leading to lacunar infarction been identified.17 Magnetic resonance angiography (MRA) can detect intracranial large artery diseases, stenoses, or occlusions in 21% of patients meeting clinical and radiologic criteria for lacunar infarcts, but only in 10% is the artery disease related to the affected penetrating vessel.18 However, studies from Asian populations have showed that small subcortical infarcts are frequently caused by branch occlusion associated with parental artery atherosclerotic plaque, or by atherosclerotic proximal small-vessel disease.19,20 These pathophysiologies are not well captured in traditional ischemic stroke classification systems.
Silent cerebral infarcts: new insights into epidemiology, mechanisms, and distal effects
MRI studies of the general population, starting to be reported from the 1990-ies and forward, have shown that most lacunar infarcts do not produce acute stroke symptoms but are clinically unrecognized or "silent."3 Silent cerebral infarcts (95% of which are "lacunar") are at least five times as common as symptomatic ones. Age and hypertension are the two factors associated with the strongest risk of SBI. Longitudinal studies suggest an annual incidence between 2% and 4%.24
Silent infarcts are not innocent; they have been shown to increase the risk of vascular events (including stroke), cognitive decline and dementia. They are "silent" only in the aspect that they have not caused acute cerebral dysfunction. Silent cerebral infarcts have the same pathological appearance as lacunar infarcts that cause acute stroke symptoms,25 suggesting that the main difference between symptomatic and silent cerebral infarcts are their size and location.
Recent findings suggest that the mechanisms of lacunes and white matter hyperintensities are intimately connected and identify the edge of white matter hyperintensities as a predilection site for lacunes.26 Duering et al. examined the spatial relationships between incident lacunes and white matter hyperintensities in 276 patients with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), The majority (n=95; 91.3%) of lacunes developed at the edge of a white matter hyperintensity whereas few lacunes were found to develop fully within or outside white matter hyperintensities. The majority of incident lacunes appeared to have developed proximal to a white matter hyperintensity along the course of perforating vessels supplying the respective brain region.
Recent findings have also linked silent brain infarcts to distal effects of focal cortical thinning in cortical regions with high probability of connectivity with the incident infarct.27 The findings provide in vivo evidence for secondary cortical neurodegeneration after subcortical ischemia as a mechanism for brain atrophy in cerebrovascular disease, and a mechanism of relevance for the cognitive effects of silent deep cerebral infarcts.
Other recent studies have documented the effects on silent small deep infarcts on white matter tract integrity and connectivity in the brain. Reijmer et al.28 demonstrated by tractography that lacunar infarcts were associated with abnormalities in the affected white matter tract that extended centimeters beyond the lesion visible on conventional MRI. Lawrence et al.29 analyzed structural connectivity between multiple cortical and subcortical brain regions in 115 patients with lacunar infarction and leucoaraiosis and 50 healthy individuals. They demonstrated that brain network connectivity in small vessel disease is disturbed, that it was related to disease severity, and could fully or in part account for observed associations between MRI measures and cognitive dysfunction related to small vessel disease.
Cerebral microbleeds
Another component of the spectrum of cerebral small vessel disease is cerebral microbleeds (CMBs). CMBs are small (2 to 5 mm) hypointense lesions on paramagnetic sensitive MR sequences such as T2*-weighted gradient-echo (GRE) or susceptibility-weighted sequences. They are most often located in the cortico-subcortical junction, deep grey or white matter in the cerebral hemispheres, brainstem and cerebellum. The common occurrence of CMB has only been recognized since the mid 1990-ies, as CMBs are generally not visualized on CT or FLAIR, T1 or T2 weighted MR sequences. There has been substantial progress in the understanding of CMBs during recent years (recently summarized in a comprehensive monograph,30 but there are several areas in need of further study. CMBs localized to the deep hemispheric regions, brain stem and cerebellum have been closely linked to traditional vascular risk factors similar to lacunar infarcts, whereas multiple, strictly lobar CMBs, has been shown to be highly specific for severe cerebral amyloid angiopathy, and is an important prerequisite to establish the diagnosis of cerebral amyloid angiopathy in life.31
Cerebral microbleeds have emerged as potential imaging markers of bleeding-prone small vessel arteriopathies, in particular small vessel disease related to hypertension and to cerebral amyloid angiopathy. A large number of association studies (outside the scope of the present review) have been published suggesting that presence of microbleeds negatively influence a large number of early and long-term outcomes after TIA, ischemic stroke, and intracerebral hemorrhage. However, these findings are not yet so firmly established that they should be used to influence clinical decision making on acute and secondary preventive therapies in TIA and stroke. A large study on the risk of cerebral microbleeds at baseline and risk of intracerebral hemorrhage during anticoagulant therapy in patients with atrial fibrillation is well in progress in the UK.
Cortical microinfarcts-the most common type of small vessel disease in the brain?
A recent addition to the spectrum of silent brain infarcts is cerebral microinfarcts, very small (<1 mm) often cortical infarcts, detected at autopsy at microscopic examination or at high-field 7T MRI.31,32 Cerebral microinfarcts may be up to 15 times more frequent than "conventional" SCIs. Their role in contributing to clinical features and prognosis is currently unclear and under study. However, microinfarcts appear to be associated with dementia even after controlling for other neuropathologies, suggesting that microinfarct burden may be an important link between small-vessel disease and cognitive impairment.33,34 A recent study estimated that even the finding of only a few microinfarcts on routine pathologic sampling indicate a likely overall burden of hundreds of these small lesions.35
Neuroimaging standards for research into small vessel disease
A report on neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration was recently published by an international consensus group from the Centres of Excellence in Neurodegeneration.13 The report provides a common advosry about terms and definitions for features visible on MRI, propose minimum standards for image acquisition and analysis, agrees on standards for scientific reporting, and review emerging imaging methods for detection and quantification of preclinical manifestations of SVD. Although the findings and recommendations apply to research studies, they can also be used in a clinical setting to standardize image interpretation, acquisition and reporting.
A highlight of the report is delineation on the variable fates of lesions related to small vessel disease and the convergence of acute lesions with different causes but similar late appearance on MRI (See above, Figure 1). Another highlight is the advice on differential diagnosis between different manifestations of small vessel disease and other pathologies (Figure 2). The report is strongly recommended and should be widely used.
Conclusions
Brain imaging is essential for the understanding of small vessel disease in the brain. Several recent advances have been made that change many previous concepts of small vessel disease. Much current research converge on identifying the full spectrum of small vessel disease imaging findings in individuals, and relate findings to prognostic features of stroke and other vascular events, disability and participation, cognition, and survival. The application of these tools and findings on large-scale studies on interventions and novel therapies is eagerly awaited.