Statins in Acute Ischemic Stroke: A Systematic Review
Article information
Abstract
Background and Purpose
Statins have pleiotropic effects of potential neuroprotection. However, because of lack of large randomized clinical trials, current guidelines do not provide specific recommendations on statin initiation in acute ischemic stroke (AIS). The current study aims to systematically review the statin effect in AIS.
Methods
From literature review, we identified articles exploring prestroke and immediate post-stroke statin effect on imaging surrogate markers, initial stroke severity, functional outcome, and short-term mortality in human AIS. We summarized descriptive overview. In addition, for subjects with available data from publications, we conducted meta-analysis to provide pooled estimates.
Results
In total, we identified 70 relevant articles including 6 meta-analyses. Surrogate imaging marker studies suggested that statin might enhance collaterals and reperfusion. Our updated meta-analysis indicated that prestroke statin use was associated with milder initial stroke severity (odds ratio [OR] [95% confidence interval], 1.24 [1.05-1.48]; P=0.013), good functional outcome (1.50 [1.29-1.75]; P<0.001), and lower mortality (0.42 [0.21-0.82]; P=0.0108). In-hospital statin use was associated with good functional outcome (1.31 [1.12-1.53]; P=0.001), and lower mortality (0.41 [0.29-0.58]; P<0.001). In contrast, statin withdrawal was associated with poor functional outcome (1.83 [1.01-3.30]; P=0.045). In patients treated with thrombolysis, statin was associated with good functional outcome (1.44 [1.10-1.89]; P=0.001), despite an increased risk of symptomatic hemorrhagic transformation (1.63 [1.04-2.56]; P=0.035).
Conclusions
The current study findings support the use of statin in AIS. However, the findings were mostly driven by observational studies at risk of bias, and thereby large randomized clinical trials would provide confirmatory evidence.
Introduction
Randomized controlled trials (RCTs) have demonstrated that statins are effective for primary and secondary stroke prevention, and the benefit of statins might be largely driven by lipid-lowering effect. Beyond lipid-lowering effect, experimental studies have shown that statins have pleiotropic effects of anti-inflammatory action, antioxidant effect, antithrombotic action and facilitation of clot lysis, endothelial nitric oxide synthetase upregulation, plaque stabilization, low-density lipoprotein (LDL) oxidation reduction, and angiogenesis [1-8]. These pleiotropic effects potentially benefit in acute ischemia of the brain and heart. In addition, animal experiments have shown angiogenesis, neurogenesis, and synaptogenesis in acute cerebral ischemia [9]. Thereby, statins are potentially neurorestorative as well as neuroprotective in acute cerebral ischemia.
In patients with acute coronary syndrome acute coronary syndrome or undergoing percutaneous coronary intervention, large observational studies, RCTs, and meta-analyses showed that statins improved the outcome [10-17]. Reflecting these evidences, the current cardiology guidelines recommend that 1) for patients with acute coronary syndrome, high-intensity statin therapy should be initiated or continued in all patients with ST elevation myocardial infarction and no contraindications (Class I; Level of Evidence B) [18], 2) statins, in the absence of contraindications, regardless of baseline LDL-C and diet modification, should be given to post-unstable angina/non-ST elevation myocardial infarction patients, including postrevascularization patients. (Class I; Level of Evidence A) [19], and 3) for patients undergoing percutaneous coronary intervention, administration of a high-dose statin is reasonable before percutaneous coronary intervention to reduce the risk of periprocedural MI (Class IIa; Level of Evidence A for statin naïve patients and LOE B for those on chronic statin therapy) [20].
Despite the anticipated benefit of statins in acute ischemic stroke (AIS), no large randomized trial has been conducted as in acute coronary syndrome. The current systematic review aims to systematically review the statin effect in AIS.
Methods
Using search terms of acute stroke and statin, 2,510 abstracts published until 31 December 2014 (including Epub ahead of print) were identified from PubMed search and reviewed by one author (Hong KS.). Then, we selected articles of human beings and AIS written in English. Manual review of references in articles identified 4 additional articles. As a results, the current systematic review included 70 articles: 30 articles of prestroke statin effect, 11 of in-hospital statin effect, 4 of statin withdrawal effect, 17 of statin effect in patients treated with thrombolysis, 8 of RCTs, 4 of prestroke statin effect on poststroke infection, and 7 studies with imaging surrogate markers (11 articles overlapped) (Figure 1).
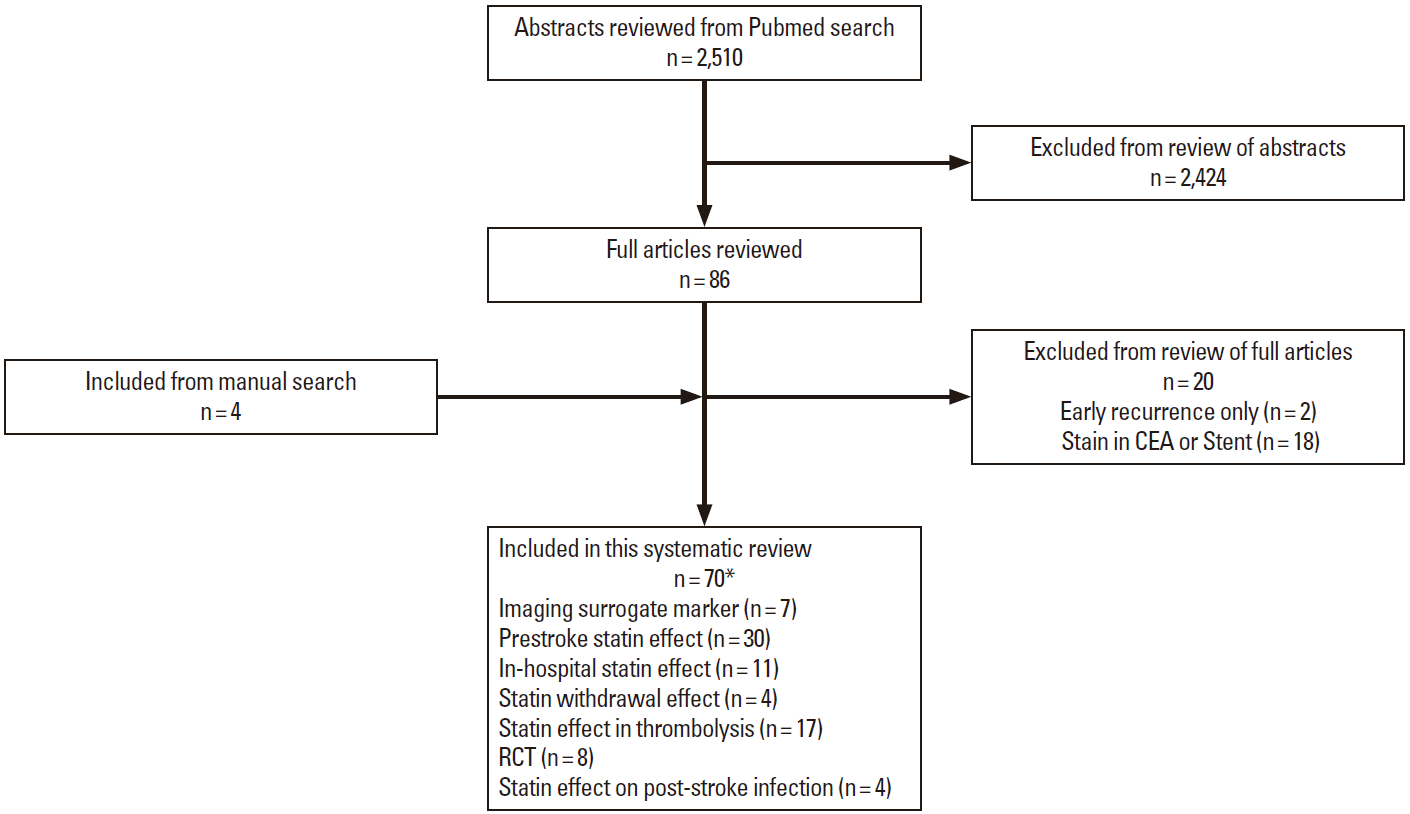
Summary of study selection. *11 articles were overlapped. CEA, carotid endarterectomy; RCT, randomized controlled trial.
For a descriptive overview, we tabulated articles according to each subject. If plausible, we conducted meta-analysis to estimate a pooled effect of statin effect in AIS. For this meta-analysis, only the original publications (excluding meta-analysis articles), which provided relevant odds ratio (OR) or hazard ratio (HR) with 95% confidence interval (CI), were included. We did not contact authors of studies to request incomplete or unpublished data. To generate a pooled-estimate using a random-effect model, we used multivariable adjusted ORs or HRs and 95% CIs. However, if adjusted ORs were not provided, unadjusted ORs were used in limited cases (1 study for prestroke statin effect on functional outcome, 2 studies for prestroke statin effect on mortality, 2 studies for prestroke statin effect on initial stroke severity, 1 study for prestroke statin effect on mortality in patients with thrombolysis, and 1 study for statin effect on post-stroke infection).
We explored for sources of inconsistency (I2) and heterogeneity. Heterogeneity was assessed by the P value of χ2 statistics and by the I2 statistics. Heterogeneity was considered significant if the P value of χ2 statistics was <0.10. For I2 statistics, we regarded I2 of <40% as minimal, 40%-75% as modest, and >75% as substantial [21]. Publication bias was assessed graphically with a funnel plot and statistically with the Begg’s test when 5 or more studies were available.
Results
Imaging surrogate marker studies
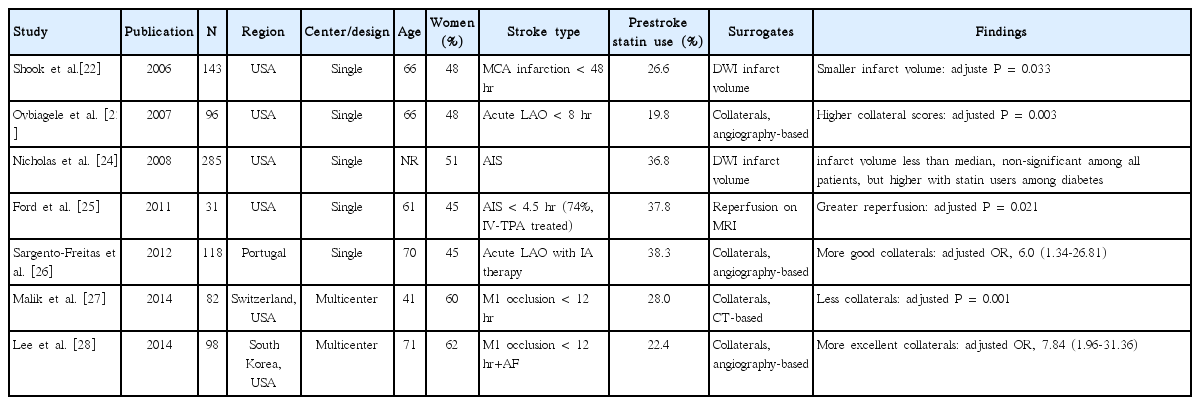
We identified 7 studies of prestroke statin effect on imaging surrogate markers in AIS: collaterals on conventional or CT angiography in 4, infarction volume on diffusion-weighted image (DWI) in 2, and reperfusion on perfusion MRI in 1 study (Table 1) [22-28]. Among the 4 studies assessing collaterals in patients with acute large artery occlusion within 8 to 12 hours [23,26-28], 1 CT-based study showed that prestroke statin was associated with less collaterals [27]. However, on the contrary, 3 conventional angiography-based studies showed that prestroke statin use was associated with more collaterals [23,26,28]. Statin might enhance collaterals by inducing endothelial nitric oxide synthase activity and angiogenesis as shown in human coronary arteries [1,2,4,29].
Prestroke statin effect on infarction volume was inconsistent across the 2 studies. In 1 study undergoing DWI evaluation within 48 hour (median time, 24 hours) of onset in patients with non-lacunar middle cerebral artery territory infarct, the prestroke statin group versus the no statin group had a significantly smaller infarct volume (median volume, 25.4 cm3 vs. 15.5 cm3, P=0.033 after adjusting covariates) [22]. In another study, prestroke statin was not associated with infarction volume [24]. However, the latter study had major limitations in that about 45% of patients had lacunar infarction and less than 40% performed DWI within 24 hours [24].
In 1 small study (n = 31) which performed serial perfusion MRIs within 4.5 hours and at 6 hours after stroke onset, prestroke statin use was associated with 2- to 3-fold greater early reperfusion in all patients as well as subgroup of intravenous tissue plasminogen activator (IV-TPA) treated patients (74%) [25]. Statin effect of enhancing collaterals, antithrombotic effect, and facilitating fibrinolysis might lead to better early reperfusion in acute cerebral ischemia [1,3,5].
Prestroke statin effect in acute ischemic stroke
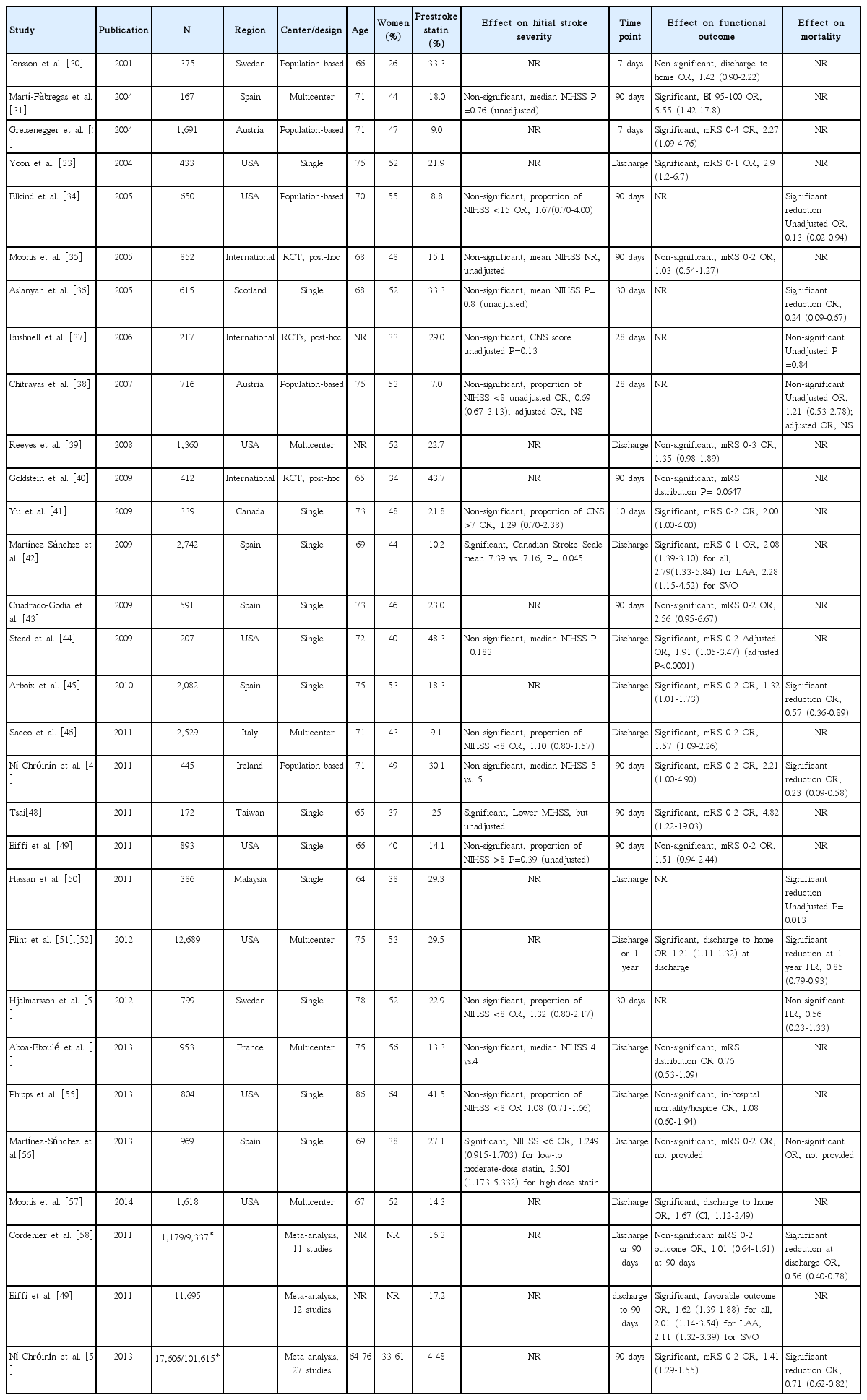
We identified 30 articles (28 original articles, 3 meta-analyses, and 1 article providing both original data and meta-analysis findings) evaluating prestroke statin effect on initial stroke severity, functional outcome or short-term mortality (Table 2) [30-59].
Prestroke statin effect on initial stroke severity
Seventeen original articles were identified and summarized in Table 2. Most studies used the NIHSS score to measure initial stroke severity except for 3 studies, but the employed analytic methods were highly variable across studies, comparing median NIHSS scores or proportion of mild stroke with variable thresholds. In 3 of the 17 studies, prestroke statin was significantly associated with milder initial stroke severity or higher proportion of mild stroke [42,48,56]. Seven studies provided ORs with 95% CI (adjusted ORs in 5 studies and unadjusted ORs in 2 studies) [34,38,41,46,53,55,56]. Pooling 7 studies involving 6,806 patients showed that prestroke statin use was associated with milder stroke severity at stroke onset (OR, 1.24; 95% CI, 1.05-1.48; P= 0.013). Heterogeneity across studies was not found (P= 0.63, I2=0%) (Figure 2A). There was no significant publication bias (P=0.322) (Supplemental Figure 1). Pooling 5 studies providing adjusted ORs also showed a significant prestroke statin effect on initial stroke severity (OR, 1.24; 95% CI, 1.04-1.48; P=0.018) (Supplemental Figure 2).
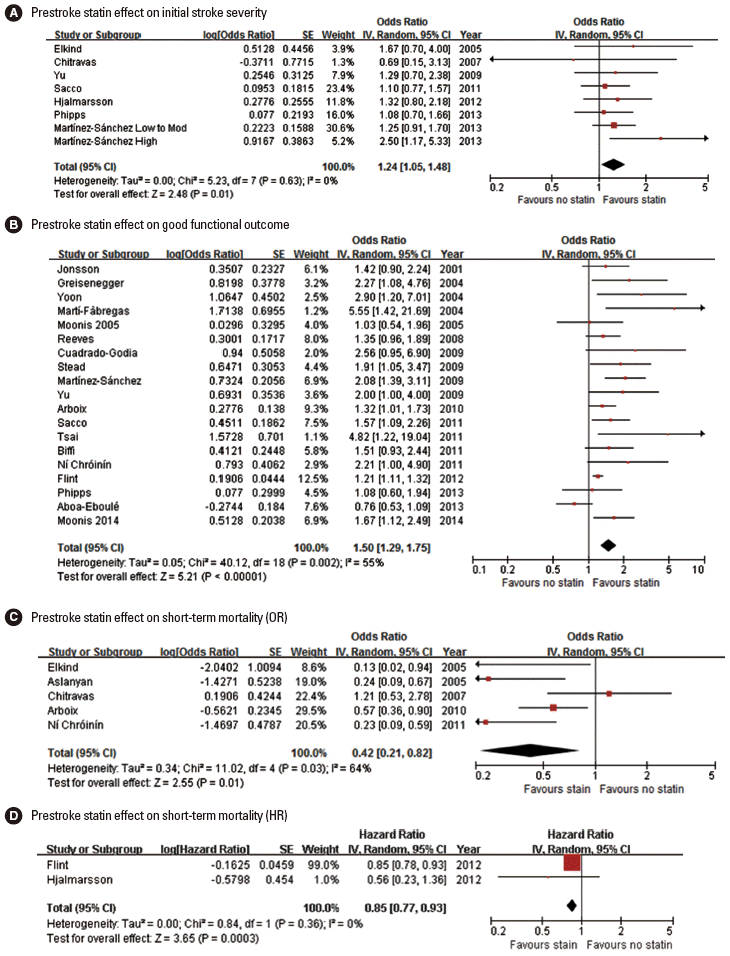
Association of prestroke statin use and initial stroke severity (A), good functional outcome (B), and short-term mortality (C, pooling studies providing OR; D, pooling studies providing HR). Values of OR or HR greater than 1.0 indicate that prestroke statin use was associated with milder initial stroke severity (A), good functional outcome (B), and higher risk of mortality (C and D). SE, standard error; IV, inverse variance; CI, confidence interval.
Prestroke statin effect on functional outcome
Three meta-analyses (outcome at 90 days outcome in 2 studies and discharge or 90 days in 1 study) [49,58,59] and 21 original articles (outcome at discharge or 7-10 days in 14 studies and at 90 days in 7 studies) [30-33,35,39-49,51,54,55,57] were identified and summarized in Table 2. For functional outcome endpoint, modified Rankin Scale (mRS) 0-2 was most widely employed as a good outcome (12 studies: 10 original articles and 2 meta-analyses). In 14 (12 original article and 2 meta-analyses) of the 24 studies, patients with prestroke statin were more likely to achieve good functional outcome. Pooling 19 original publications (involving 30,942 patien ts) [30-33,35,39,41-49,51,54,55,57], which provided adjusted ORs (95% CI), showed that prestroke statin use was associated with good functional outcome (OR, 1.50; 95% CI, 1.29-1.75; P<0.001). There was a significant and modest heterogeneity across the studies (P=0.002, I2=55%). However, the heterogeneity was related to the magnitude of effect rather than the direction of effect (Figure 2B). A significant publication bias was found (P=0.001), but it was mainly attributed to studies with relatively small sample sizes (Supplemental Figure 3). Regarding ischemic stroke subtypes, 1 meta-analysis showed that the association of prestroke statin use and good functional outcome was significant in patients with large artery atherosclerosis and small vessel occlusion, but not in cardioembolic stroke [49].
Prestroke statin effect on short-term mortality
Two meta-analyses (90-day mortality) [58,59] and 10 original articles (mortality at discharge in 3 studies, 20-30 days in 4 studies, 90 days in 2 studies, and 365 days in 1 study) [34,36-38,45,47,50,52,53,56] were identified and summarized in Table 2. In 8 (6 original articles and 2 meta-analyses) of the 12 studies, patients with prestroke statin had a lower mortality. Pooling 5 original publications (involving 4,508 patients), which provided ORs with 95% CI (adjusted ORs in 3 studies [36,45,47] and unadjusted ORs in 2 studies [34,38]), showed that prestroke statin use was associated with lower mortality (OR, 0.42; 95% CI, 0.21-0.82; P=0.011). A significant and modest heterogeneity across the studies was found (P=0.03, I2=64%), but the treatment effect was in the same direction except for 1 study (Figure 2C). There was no significant publication bias (P=0.624) (Supplemental Figure 4). Pooling 3 studies providing adjusted ORs also showed a significant association of prestroke statin use with reduced mortality (OR, 0.36; 95% CI, 0.18-0.70; P=0.003) (Supplemental Figure 5). Two studies (involving 13,488 patients) reported adjusted HRs instead of ORs [52,53]. Pooling these 2 studies also showed that prestroke statin use was associated with lower mortality (HR, 0.85; 95% CI, 0.77-0.93; P=0.0003). There was no significant heterogeneity across the studies (P=0.36, I2=0%) (Figure 2D).
In-hospital statin effect in acute ischemic stroke
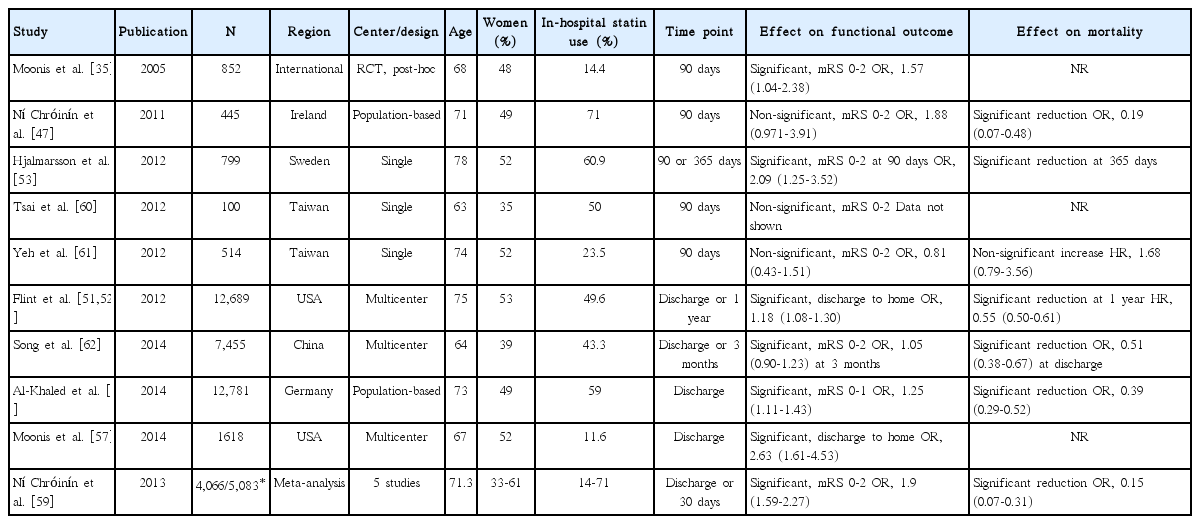
We identified 11 articles (10 original articles and 1 meta-analysis) that assessed the in-hospital statin effect on functional outcome or short-term mortality (Table 3) [35,47,51-53,57,59-63].
In-hospital statin effect on functional outcome
One meta-analysis (at discharge or 30 days) [59] and 9 original articles (at discharge in 4 studies and at 90 days in 5 studies) [35,47,51,53,57,60-63] assessed the in-hospital statin effect on functional outcome (Table 3). For functional outcome endpoint, mRS 0-2 outcome was most commonly used as a good functional outcome (in 7 studies: 6 original articles and 1 meta-analysis). In 7 (6 original article and 1 meta-analysis) of the 10 studies, patients with in-hospital statin had a better functional outcome. Pooling 8 studies (involving 37,153 patients) [35,47,51,53,57,61-63], which provided adjusted ORs (95% CI), showed that in-hospital statin use was associated with good functional outcome (OR, 1.31; 95% CI, 1.12-1.53; P=0.001). There was a significant and modest heterogeneity across the studies (P=0.005, I2=65%), but the treatment effect was generally in the same direction except for 1 study (Figure 3A). There was no significant publication bias (P=0.322) (Supplemental Figure 6).

Association of in-hospital statin use and good functional outcome (A), and short-term mortality (B, pooling studies providing ORs; C, pooling studies providing HRs). Values of OR or HR greater than 1.0 indicate that in-hospital statin use was associated with good functional outcome (A), and higher risk of mortality (B and C). SE, standard error; IV, inverse variance; CI, confidence interval.
In-hospital statin effect on short-term mortality
One meta-analysis (at discharge or 30 days) and 6 original articles (at discharge in 2 studies, 90 days in 2 studies, and 365 days in 2 studies) assessed the in-hospital statin effect on shortterm mortality (Table 3). Of the 7 studies, 6 studies (5 original article and 1 meta-analysis) showed that patients with in-hospital statin use had a significantly lower mortality, whereas 1 study reported non-significant increase in mortality with in-hospital statin use. Three original articles provided adjusted ORs with 95% CI, and pooling these 3 studies involving 20,681 patients showed that in-hospital statin use was associated with lower mortality (OR, 0.41; 95% CI, 0.29-0.58; P<0.001). A non-significant and modest heterogeneity across the studies was found across the studies (P= 0.12, I2=53%) (Figure 3B). Three original articles provided adjusted HRs with 95% CI, and pooling these 3 studies involving 14,002 patients showed that in-hospital statin use was not significantly associated with lower mortality (HR, 0.62; 95% CI, 0.33-1.16; P=0.138). A significant and substantial heterogeneity across the studies was found (P=0.002, I2=84%) (Figure 3C).
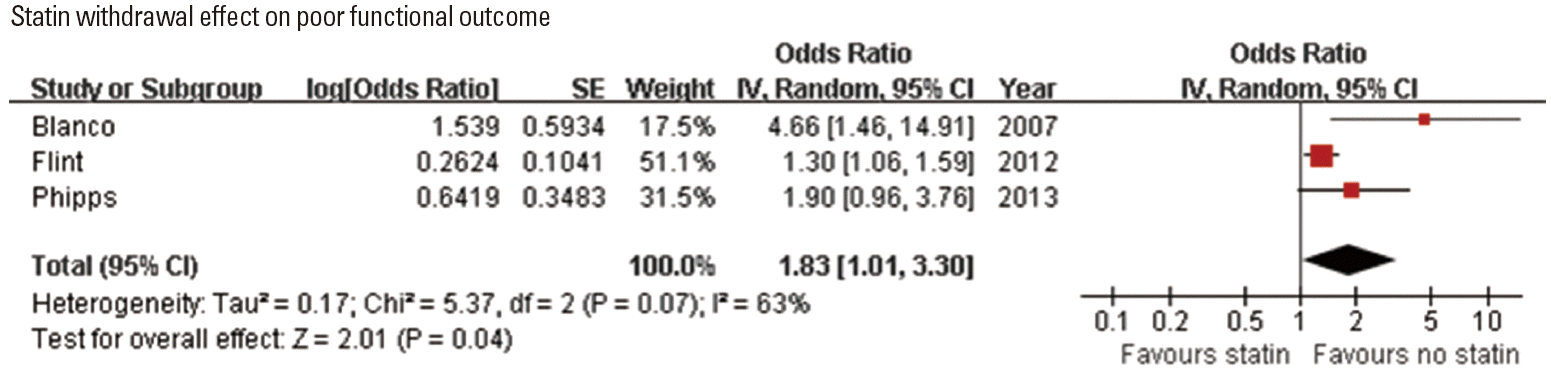
Statin withdrawal effect in acute ischemic stroke
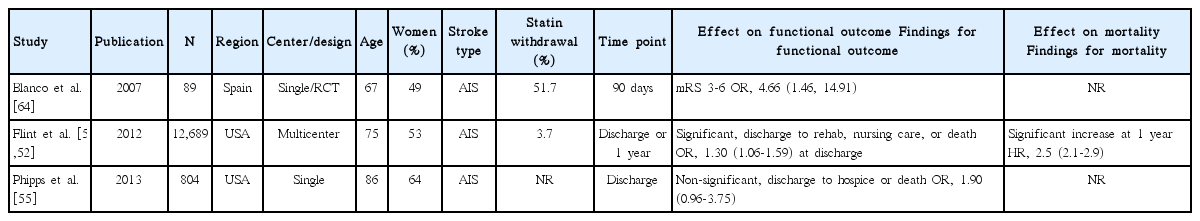
Statin withdrawal effect was tested in one RCT performed in a single center [64], and explored in three observational studies (Table 4) [51,52,55]. Of 3 studies assessing functional outcome (adjusted ORs for 90-day mRS 3-6 outcome in one study and for poor discharge disposition in 2 studies) [51,55,64], 2 studies showed that statin withdrawal was associated with poor outcome [51,64]. Pooling the 3 studies involving 13,583 patients showed that statin withdrawal was associated with poor functional outcome (OR, 1.83; 95% CI, 1.01-3.30; P=0.045). A significant and modest heterogeneity across the studies was found (P=0.07, I2=63%) (Figure 4). In one study, statin withdrawal was associated with an increased risk of 1-year mortality (HR, 2.5; 95% CI, 2.1-2.9; P<0.001) [52].
Statin effect in patients with thrombolysis
We identified 17 studies (15 original articles, 4 meta-analyses, and 2 article providing both original data and meta-analysis findings) exploring statin effect on functional outcome, mortality, or symptomatic hemorrhagic transformation (SHT) in patients with thrombolysis (IV-TPA only in 12 studies, intra-arterial thrombolysis [IA] only in 2 studies, and IV-TPA or IA in 3 studies) (Table 5) [58,59,65-79].
Statin effect on functional outcome in patients with thrombolysis
Fifteen studies (14 original articles, 3 meta-analyses, and 2 article providing both original data and meta-analysis findings) reported the statin effect on functional outcome [59,65,67-74,76-79]. Good functional outcome was defined as mRS 0-1 in 6 studies and mRS 0-2 in 8 studies, and mix-up of variable criteria in 1 meta-analysis. In earlier 3 meta-analyses [59,73,75], statin was not associated with good functional outcome, whereas 5 studies among the 14 original articles showed that statin was associated with good functional outcome [65,67,72,76,78]. Pooling 11 original articles (involving 10,876 patients, 1 article providing 2 ORs for both statin before and after and statin after only) [65,67,69-74,76,78,79], which provided adjusted ORs with 95% CI, showed that statin use in patients treated with thrombolysis was associated with good functional outcome (OR, 1.44; 95% CI, 1.10-1.89; P=0.008). A significant and modest heterogeneity was found across the studies (P<0.001, I2=67%), but the direction of treatment effect was generally consistent except for 2 studies (Figure 5A). There was no significant publication bias (P=0.493) (Supplemental Figure 7).

Association of statin use and good functional outcome (A), 90-day mortality (B), and symptomatic hemorrhagic transformation (C). Values of ORs than 1.0 indicate that statin use was associated with good functional outcome (A), higher risk of mortality (B), and higher risk of symptomatic hemorrhagic transformation (C). SE, standard error; IV, inverse variance; CI, confidence interval.
Statin effect on mortality in patients with thrombolysis
Statin effect on the 90-mortality in patients with thrombolysis was assessed in 2 meta-analyses and 6 original articles (Table 5) [58,59,68,71,73,74,76,79]. Among the 3 meta-analyses, 1 meta-analysis showed that statin use was associated with increased mortality [59]. Of the 6 original articles, 1 study showed that, statin use was significantly associated with lower mortality [76], but the other studies found no significant effect. Pooling 5 original articles (involving 8,237 patients) [71,73,74,76,79], which provided ORs with 95% CI (adjusted OR in 4 studies and unadjusted OR in 1 study), showed that statin use in patients treated with thrombolysis neither increased nor decreased mortality (OR, 0.87; 95% CI, 0.58-1.32; P=0.518). A significant and modest heterogeneity across the studies was found (P=0.02, I2=65%) (Figure 5B). There was no significant publication bias (P=0.142) (Supplemental Figure 8). Pooling 4 studies providing adjusted ORs also showed that statin use was not associated with mortality in patients with thrombolysis (OR, 0.77; 95% CI, 0.48-1.25; P=0.289) (Supplemental Figure 9).
Statin effect on symptomatic hemorrhagic transformation in patients with thrombolysis
Sixteen studies (15 original articles, 3 meta-analyses, and 2 article providing both original data and meta-analysis findings) reported the statin effect on SHT (Table 5) [58,65-79]. In 2 [58,75] of the 3 meta-analyses and 3 [68,72,78] of the 15 original articles, statin use was associated with an increased risk of SHT. Pooling 9 original articles (involving 10,419 patients) [67,68,70-74,76,78], which provided adjusted ORs with 95% CI, showed that statin use in patients treated with thrombolysis was associated with an increased risk of SHT (OR, 1.63; 95% CI, 1.04-2.56; P=0.035). A significant and modest heterogeneity across the studies was found (P=0.003, I2=65%) (Figure 5C). There was no significant publication bias (P=0.655) (Supplemental Figure 10).
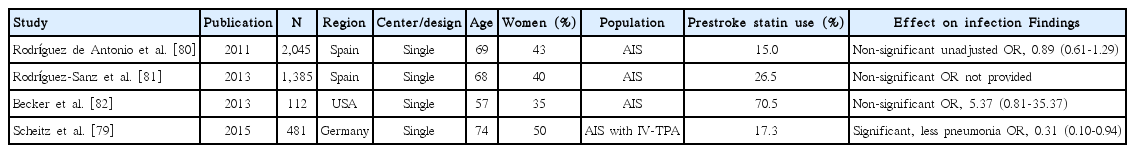
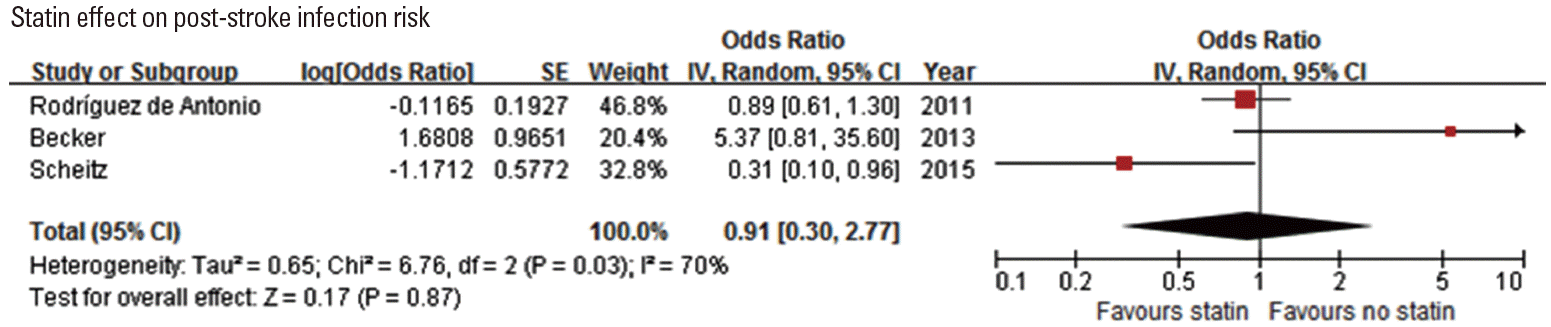
Statin effect on post-stroke infection
Since statins have immunomodulatory effect, several studies assessed the association of statin use with post-stroke infection. Among 4 studies [79-82], 1 study [79] showed that post-stroke pneumonia was less frequent in patients with prestroke statin use among IV-TPA treated patients (Table 6). Pooling 3 original articles (involving 2,638 patients) [79,80,82], which provided ORs with 95% CI (adjusted OR in 2 studies and unadjusted OR in 1 study), showed that the effect of statin on post-stroke infection was not significant (OR, 0.91; 95% CI, 0.30-2.77; P=0.867). There was a significant and modest heterogeneity across the studies (P=0.03, I2=70%) (Figure 6). Pooling 2 studies providing adjusted ORs also showed that statin use was not associated with post-stroke infection (OR, 1.16; 95% CI, 0.07-18.87; P=0.916) (Supplemental Figure 11).
Randomized controlled trials
The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) study randomized patients at 1 to 6 months after stroke. Therefore, the trial results cannot be considered as a reliable guide for statin use in AIS. Literature search identified 6 RCTs (statin withdrawal effect on functional outcome in 1, statin effect on functional outcome in 1, recurrent stroke within 90 days in 1, early neurological improvement in 1, and surrogate markers in 2 studies) [64,83-87], and one meta-analysis (Table 7) [88]. In addition, one phase 1B dose-finding single arm trial using an adaptive design of increasing lovastatin up to 10 mg/kg/day assessed the safety of high-dose statin, which showed that the final model-based estimate of toxicity was 13% (95% CI 3%-28%) for a dose of 8 mg/kg/day [89]. There has been no large RCT, and the sample sizes of the published RCTs were small (ranging between 33 and 392), not adequately powered to assess the statin effect in AIS.
Markers of Inflammation after Simvastatin in Ischemic Cortical Stroke (MISTICS) was a pilot, double-blind, randomized, multicenter clinical trial, comparing inflammatory biomarkers between simvastatin versus placebo in patients with cortical AIS [84]. The trial failed to demonstrate the anti-inflammatory effect of statin in human stroke despite rapid and sustained reduction of total and LDL-C levels with simvastatin. For clinical endpoints, patients on simvastatin compared to those on placebo were more likely to achieve NIHSS improvement 4 or more at 3 days, but did not achieve better 90-day mRS outcome.
Fast Assessment of Stroke and Transient Ischemic Attack to Prevent Early Recurrence (FASTER) was a relatively large RCT comparing simvastatin 40 mg versus placebo in 392 patients with a transient ischemic attack or minor stroke within the previous 24 hours [83]. Because of the slow enrollment rate, the trial was early terminated after enrolling only 392 patients and thereby substantially underpowered. The rate of the primary endpoint of recurrent stroke within 90 days was 10.6% for the simvastatin group versus 7.3% for the placebo group (relative risk [RR], 1.3; 95% CI, 0.7-2.4; P=0.64).
Although the benefit of early statin initiation in AIS has not been demonstrated in RCTs, the harmful effect of statin withdrawal was demonstrated in a small, single center, randomized trial [64]. In the trial, 89 patients with prestroke statins and hemispheric ischemic stroke within 24 hours were randomized to either transient statin withdrawal for the first 3 days or to continuation of statin treatment with atorvastatin 20 mg daily. The withdrawal group versus the continuation group was more likely to have poor functional outcome at 90 days (mRS 3-6, primary endpoint) (60.0% vs. 39.0%; adjusted OR [95% CI], 4.66 [1.46 to 14.91]), to experience early neurological deterioration of worsening NIHSS score 4 or more (65.2% vs. 20.9%; adjusted OR [95% CI], 8.67 [3.05 to 24.63]), and to have a greater infarct volume increase between 4 and 7 days. Based on this trial results, the American Stroke Association guidelines recommend the continuation of statin therapy during the acute period among patients already taking statins at the time of ischemic stroke (Class IIa; LOE B). However, the American Stroke Association guidelines do not provide specific recommendations regarding when to start statins in AIS patients with no prior statin treatment [90].
In a recent meta-analysis including 7 published and unpublished RCTs involving 431 patients with AIS or transient ischemic attack within 2 weeks, all-cause mortality did not differ between the statin and placebo groups (OR 1.51, 95% CI 0.60 to 3.81) [88].
Discussion
Our systematic review could not find the evidence of statin benefit in AIS from RCTs although a small RCT demonstrated the harm of statin withdrawal. The results from observational studies were inconsistent. However, our updated meta-analysis using available data from original publications suggests that 1) prestroke statin use might reduce stroke severity at stroke onset, functional disability, and short-term mortality, 2) immediate post-stroke statin treatment might reduce functional disability and short-term mortality, whereas statin withdrawal might lead to worse outcome, and 3) in patients treated with thrombolysis, statins might improve functional outcome despite of an increased risk of SHT.
Imaging surrogate maker studies would provide a proof-of-concept for potential mechanisms of statin benefit in AIS. In general, surrogate marker studies suggest that statin benefit might be mediated by more collaterals and better reperfusion in AIS, and the findings in human with AIS are consonant with animal experiment study findings of improved cerebral flow secondary to upregulation of endothelial nitric oxide synthase, enhanced fibrinolysis, and reducing infarct size with statin treatment [3-5].
Investigating the prestroke statin effect would be a useful approach to assess the neuroprotective effect of statins in AIS. However, an RCT testing prestroke statin effect is not practically feasible because it requires a tremendous sample size. Given the neuroprotective effect of statins from animal experiment studies and imaging surrogate marker studies in human stroke, prestroke statin might limit ischemic brain damage and lead to mild stroke severity, and this effect, in part, might contribute to the better functional outcome with prestroke statin use. However, most published articles failed to show that prestroke statin was associated with less severe stroke. Since the statin benefit would not be substantial, the small sample sizes of the most published articles might account for the negative results. Previously, no meta-analysis has explored this statin effect. In our meta-analysis including data from 6,806 patients, pre-stroke statin use was associated with a 1.24-fold greater odds of stroke with milder severity, suggesting statin’s neuroprotective effect during acute cerebral ischemia in human. A recent Korean large retrospective study (n=8,340) using propensity score matching analysis showed that prestroke stroke use was associated with mild stroke severity at presentation [91].
An earlier post-hoc analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels study explored statin effect on functional outcome in patients with recurrent stroke (i.e., prestroke statin effect on recurrent stroke), which had an advantage of randomizing patients either to statin or placebo. The authors suggested that the outcome of recurrent ischemic cerebrovascular events might be improved among statin users as compared with patients on placebo. However, the result was significant for the overall cohort, including patients with event-free as well as those with recurrent stroke. The analysis restricted patients with recurrent ischemic stroke outcome did not show a significant benefit of statin on functional outcome [40].
For the statin effect on functional outcome, 2 previous meta-analyses (patients included in functional outcome analysis: n=11,965 in one study by Biffi et al. [49] and n = 17,152 in another by Ní Chróinín et al. [59]) showed the association of prestroke statin use with good functional outcome. In accord with previous results, the finding of our updated meta-analysis including more patients (n=30,942) strongly suggests that prestroke statin use might improve functional outcome. It is unclear whether, in addition to neuroprotection during ischemia, statin’s facilitation of recovery after stroke as shown in animal experiments [9], leads to the better functional outcome. In a recent large observational study in Korea, even after adjusting initial stroke severity as well as other covariates, prestroke statin users compared to non-users were more likely to achieve good outcome (mRS 0-2 outcome) at discharge, suggesting statin’s dual effect of neuroprotection and neurorestoration [91].
Our updated meta-analyses showed that statin whether administered prior to stroke or immediately after stroke was associated with better survival, as observed in earlier meta-analyses [58,59]. In addition to better functional outcome, preventing recurrent vascular event might account for the statin benefit of reducing short-term mortality. In a meta-analysis of 7 RCTs in patients with acute coronary syndrome, statin initiation during acute period was associated with reduced mortality [92]. Therefore, statin therapy might have beneficial effect of reducing mortality in patients with acute ischemia in the brain as well as in the heart. In patients with recent acute coronary syndrome, high intensity statin versus moderate intensity statin had a greater benefit in reducing mortality [93]. For patients with AIS, a large observational study showed that 1-year survival benefit with statin during AIS was greater with high-dose statins than with low-dose statins [52]. However, there has been no evidence from RCTs.
Worse outcome with statin withdrawal in a small RCT might indirectly indicate the statin benefit in AIS [64]. Supporting the RCT finding, a large observational study [51] and our meta-analysis showed the harmful effect of statin withdrawal during AIS. In the RCT, statin withdrawal even for a brief period of 3 days led to early neurological deterioration and greater infarct volume increase as well as 90-day worse functional outcome. Therefore, the potential mechanisms might be related to, rather than LDL-lowering, pleiotropic effects on endothelial function, inflammation, platelet, and fibrinolytic system [1,3-5].
Statins have antithrombotic and fibrinolytic effects, and in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels trial patients on high-dose atorvastatin compared to those on placebo had more hemorrhagic strokes. Therefore, among neurologists, there has been concern on the increased risk of hemorrhagic transformation with statin use in AIS, particularly for patients treated with thrombolysis. The current meta-analysis showed that statin was associated with an increased risk of SHT after thrombolysis, as shown in a previous meta-analysis [58]. However, despite the increased risk of SHT, the functional outcome was better with statin use in our meta-analysis, which was not observed in earlier meta-analyses [59,73,75]. Therefore, prestroke statin use would not be a contraindication for thrombolysis. However, whether statin therapy should be initiated immediately after reperfusion therapy in AIS as in acute coronary syndrom needs to be tested with RCTs.
This study has several limitations. Our findings were almost exclusively driven from data of observational studies, which are at risk of bias. In most outcomes, we found a large amount of heterogeneity in the results among the included studies. However, in general, the heterogeneity was brought by the magnitude of effect rather than the direction of effect. For several outcomes, unadjusted ORs as well as adjusted ORs were combined to generate pooled estimates. However, unadjusted ORs used were from limited articles with relatively small sample sizes. Therefore, the effect on our findings was not substantial as shown in additional analyses pooling adjusted ORs only. The current study did not assess the statin effect on early recurrent stroke, which would be of interest of topics on future investigation. Finally, if the literature search fails to find out all relevant articles, the result of meta-analysis is at risk of bias. As we only searched PubMed, several relevant articles might be missed. However, to minimize this risk, we performed additional hand search by reviewing references listed in the included original publications and meta-analyses.
In conclusion, the current systematic review supports the benefit of statins in AIS. However, the findings were largely driven by observational studies, and thereby the benefit needs to be confirmed by well-designed, large RCTs.
Notes
Hong KS has received lecture honoraria from Pfizer Korea related to the current topic.
The authors have no financial conflicts of interest.