Anterior Optic Pathway Compression Due to Internal Carotid Artery Aneurysms: Neurosurgical Management and Outcomes
Article information
Abstract
Background and Purpose
Compression of the anterior optic pathway results in visual deficits that can lead to the detection of unruptured aneurysms in the internal carotid artery (ICA). The general types of treatment modalities for aneurysms and visual deficits include surgery and endosaccular coiling. This study retrospectively analyzed and compared the resolution of visual deficits following surgery or endosaccular coiling.
Methods
We reviewed data on 33 patients with unruptured ICA aneurysms who presented with visual field deficits caused by mass effects over the anterior optic pathway. Statistical analyses were performed to identify the variables associated with the recovery of visual symptoms.
Results
Eighteen patients underwent aneurysm clipping, 2 underwent bypass surgery with endovascular trapping, and 2 underwent endovascular trapping without bypass surgery (group A). Ten patients received endosaccular coiling (group B). The visual outcomes included the following: in group A, 17 patients (73.9%) demonstrated improvement and 6 patients (26.1%) demonstrated no changes or worse outcomes; in group B, 2 patients (20.0%) demonstrated improvement and 8 patients (80.0%) demonstrated no changes or worse outcomes. Group A was associated with a higher rate of favorable outcome than group B (P = 0.007). According to the multivariate analysis, treatment without endosaccular coiling (group A) was the only variable significantly associated with improvement of visual outcome (P = 0.005; OR = 28.523; 95% CI = 2.683-303.171).
Conclusions
Treatment modality was the only predictor of improvement in visual deficits. Treatment without endosaccular coiling resulted in visual improvement significantly more often in comparison with endosaccular coiling.
Introduction
Cranial nerve deficit is a clinical condition that can lead to the detection of unruptured intracranial aneurysm (UIA). The International Study of Unruptured Intracranial Aneurysms Investigators reported that 15.4% of patients with UIAs present with cranial nerve deficits [1], and other studies report incidences of 2.2% and 8.8% [2,3]. Oculomotor nerve palsy related to posterior communicating artery aneurysm is one of the most common cranial nerve deficits caused by UIAs [2,4-9], but, less commonly, visual deficits related to UIAs that originate from the internal carotid artery (ICA) have also been reported [5,9-14].
Because the intradural course of the ICA is close to the anterior optic pathway, some UIAs that arise in this location result in some degree of visual deficit [10,11,14]. The possible mechanisms for visual deficits include the direct mass effects of the aneurysm on the anterior optic pathway, compression of the optic nerve against the bony structure by the aneurysm, and compromised vascular supply to the anterior optic pathway [10,12,14].
Neurosurgical management should be considered because the risk of rupture is higher in patients with symptomatic UIAs than asymptomatic UIAs, and management can alleviate symptoms and prevent rupture [10,14-16]. There are two general types of treatment modalities for UIAs: surgery and endosaccular coiling. Both of these treatment modalities effectively prevent UIA rupture. However, few studies report which treatment modality more effectively alleviates the visual deficits caused by UIAs that arise in the ICA. In our current study, we retrospectively analyzed and compared the results of treating UIAs that arose in the ICA following surgery or endosaccular coiling.
Methods
Study population
This study was approved by the Institutional Review Board before data collection. We retrospectively reviewed patients with UIAs in the ICA who were treated at our institution between January 2009 and March 2014. Patients were assessed according to the following inclusion criteria: 1) presented with visual dysfunction and 2) compressive optic neuropathy was confirmed using high-resolution magnetic resonance image (MRI) and ophthalmologic examination. Patients with other possible causes, such as cerebral infarction, intracerebral hemorrhage, tumorous lesions, or ophthalmologic causes (e.g., glaucoma, cataracts), were excluded. Among the patients with UIAs in the ICA, 33 patients met the inclusion criteria. Visual field examinations were performed preoperatively and ≥ 6 months after treatment. Patient characteristics, aneurysm size, location, radiological features, degree and involved location of visual dysfunction, and changes in visual symptoms after treatment were analyzed. Visual symptoms were mainly specified according to visual field impairments, and deterioration in visual acuity was only used as the reference criteria. To assess the degree of visual dysfunction, we scored the involved quadrants on a scale of 1-8 (i.e., the total number of the quadrants that demonstrated visual field defects). According to the patterns of the visual field defects and the results of high-resolution MRI, the involved locations were categorized as present in the optic nerve, chiasm, or tract.
Treatment modalities
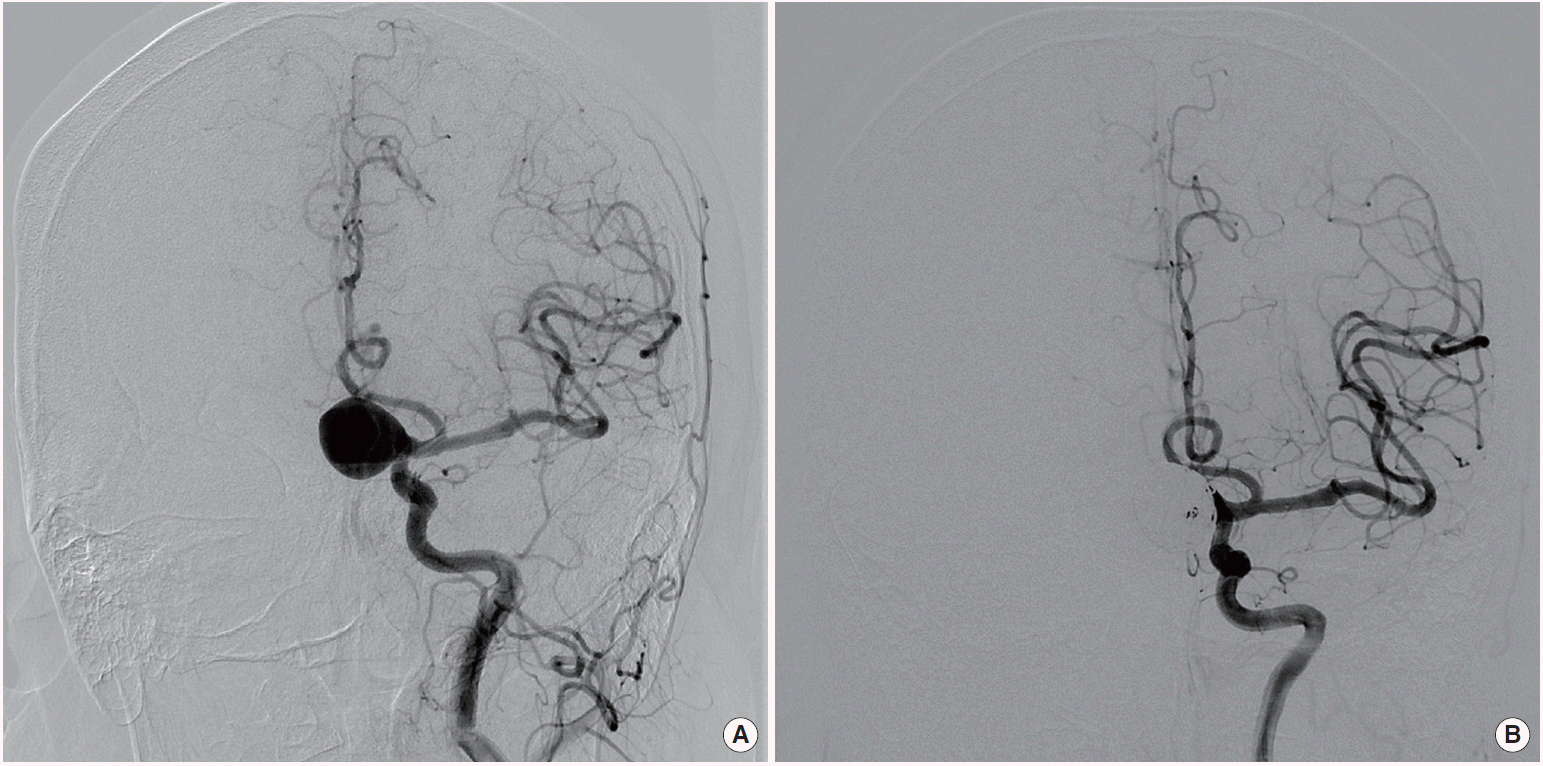
All patients were treated using surgical clipping or endovascular treatment according to interdisciplinary consensus. Direct neck clipping with or without suction decompression technique was performed on 18 patients (Figure 1). Five patients received endovascular parent artery trapping without endosaccular embolization. Extracranial-intracranial bypass surgery was performed on 3 of 5 patients before trapping due to insufficient collateral blood flow (Figure 2). These 23 patients were assigned to treatment group A (non-endosaccular embolization group), which reduces aneurysmal pulsatility and mass effects. The other 10 patients received endosaccular embolization with or without the stent-assisted technique and were assigned to treatment group B, which reduces aneurysmal pulsatility without removing mass effects (Figure 3).

(A) High-resolution magnetic resonance image (MRI) showing optic chiasm compression due to a giant aneurysm, which is located in the ophthalmic segment of the left internal carotid artery (ICA). (B) Three-dimensional (3D) angiography of the aneurysm. (C) Intraoperative photograph of the aneurysm. (D) Direct neck clipping was performed with suction decompression technique after extradural anterior clinoidectomy. 3D angiography after microsurgery; aneurysm (black arrows), left ICA (black large arrow head), and optic nerve (*).

(A) Transfemoral cerebral angiography showing a giant aneurysm located in the ophthalmic segment of the right internal carotid artery (ICA). (B) Endovascular parent artery trapping was performed after high flow bypass surgery using a radial artery graft; radial artery graft (black arrows).
Clinical and visual follow-up
Outcomes were stratified according to treatment group. The mean ± standard deviation [SD] clinical follow-up period was 21.4 ± 11.6 months (median = 22 months; range = 6-45 months). The mean follow-up period for visual field examinations was 10.4 ± 6.0 months (median = 10 months; range = 6-30 months). Treatment-related complications were also assessed during the follow-up period.
Statistics analysis
Statistical analyses were conducted using standard commercial software (IBM SPSS statistics for windows, Version 20.0, Armonk, NY: IBM Corp.). Fisher’s exact test was used for categorical variables, and Student’s t test was used for continuous variables in order to compare characteristics and treatment outcomes of group A and B. Student’s t test also used for continuous variables in order to compare characteristics of favorable outcome (improved) group and unfavorable outcome (stationary and aggravated) group. In these studies, P< 0.05 is considered statistically significant.
A multivariate analysis was performed to find independent predictors of favorable outcome using logistic regression analysis. Variables with P< 0.05 according to the univariate analysis, and variables that affected visual outcomes according to previous studies (e.g., aneurysm size, symptom duration, intra-aneurysmal thrombosis) were included in the multivariate analysis [14,17].
Results
Characteristics of the patients and aneurysms
The study population consisted of 5 men and 28 women (mean age = 60.3 ± 8.7 years; range = 43-77 years). The maximum diameter of the aneurysms ranged between 7.1-42 mm (mean = 19.8 ± 8.7 mm). Twenty-six aneurysms (78.8%) were larger than 10 mm and, among these, 6 aneurysms (18.2%) were classified as giant aneurysms ( > 25 mm). Almost all aneurysms (31 of 33) were located in the ophthalmic segment of the ICA. Only 2 giant aneurysms that were located in the cavernous segment of the ICA presented with visual disturbance. The mean number of involved quadrants was 3.4 ± 1.7 (range = 1-7), and the mean ± SD duration of visual symptoms was 6.5 ± 5.1 months (range = 1-24 months). Intra-aneurysmal thrombosis was observed in 5 patients. The sex ratio, patient age, aneurysm size, duration of visual symptoms, involved quadrant, involved location, intra-aneurysmal thrombosis and complications related to the treatment were not significantly different between treatment groups (Table 1).
Treatment outcomes
The mean follow-up period for visual field examinations after treatment was 9.6 ± 4.0 months in group A in comparison with 16.1 ± 7.5 months in group B (P= 0.026). The mean numbers of involved quadrants before treatment and at the final visual field examination after treatment were 3.7 ± 1.6 and 2.0 ± 2.3 in group A in comparison with 2.9 ± 2.0 and 3.1 ± 2.6 in group B. Improvement in visual symptoms after treatment was achieved in 17 patients in group A (73.9%) in comparison with only 2 patients in group B (20.0%). There were no changes in the visual symptoms in 4 patients in group A (17.4%) and 4 patients in group B (40.0%). Visual symptoms are aggravated in 2 patient in group A (8.7%) in comparison with 4 patients in group B (40.0%). There is a significant difference of treatment outcomes between two groups (P= 0.011; determined using the Fisher exact test) (Table 2). In addition, if the treatment outcomes are categorized into two groups, favorable outcome (improved) and unfavorable outcome (stationary and aggravated), there is also a significant difference of treatment outcomes between group A and B (P= 0.007; determined using the Fischer exact test).
Treatment-related complications
Three of 23 patients in group A (13.0%) developed treatmentrelated complications. Two patients developed transient oculomotor nerve palsy after aneurysm clipping. The other patient with a right ICA aneurysm developed left hemiparesis 2 days after endovascular parent artery trapping following extracranial-intracranial bypass surgery. Diffusion-weighted MRI revealed multifocal acute infarctions in the right anterior and middle cerebral artery territories.
One of 10 patients in group B (10.0%) developed left hemiparesis 1 month after performing endosaccular coiling on the right ICA aneurysm with stenting. Multifocal small infarctions and decreased perfusion in the right middle cerebral artery territory were revealed using MRI. transfemoral cerebral angiography demonstrated severe stenosis in the ICA due to thrombosis in the stent. The patient underwent chemical thrombolysis using Tirofiban.
Comparisons between favorable outcome group and unfavorable outcome group, and factors predictive of improved visual symptoms
The mean follow-up period for visual field examinations after treatment was 9.5 ± 3.7 months in favorable outcome group in comparison with 14.3 ± 7.4 months in unfavorable outcome group (P= 0.033). And, the mean numbers of involved quadrants at the final visual field examination after treatment was 0.7 ± 0.9 in favorable outcome group in comparison with 4.6 ± 2.0 in unfavorable outcome group (P< 0.001). Patients with favorable outcomes presented with a mean aneurysm size of 17.8 ± 7.7 mm in comparison with 20.3 ± 10.0 mm among patients with unfavorable outcomes (P= 0.411). The mean age of the patients who achieved favorable outcomes was 59.4 ± 8.2 years in comparison with 61.6 ± 9.5 years for patients with unfavorable outcomes (P= 0.480). The mean symptoms duration (5.3 ± 5.2 months for patients with favorable outcomes vs 8.4 ± 4.3 months for patients with unfavorable outcomes; P= 0.096) and the mean number of involved quadrants (3.1 ± 1.4 quadrants in patients with favorable outcome vs. 3.9 ± 2.1 quadrants in patients with unfavorable outcomes; P= 0.214) were not predictive of favorable outcomes. Intra-aneurysmal thrombosis and the involved location (e.g., optic nerve, chiasm, or tract) did not demonstrate any differences between patients with favorable and unfavorable outcomes (Table 3). For the univariate analysis, the treatment modality ‘group A’ was identified as a variable associated with favorable outcomes (P= 0.008).
A multivariate analysis was performed to find independent predictors of favorable outcome. The variable, treatment modality ‘group A’ and variables that affected visual outcomes according to previous studies (e.g., aneurysm size, symptom duration, intraaneurysmal thrombosis) were included in the multivariate analysis [14,17]. Of these variables, only the treatment modality ‘group A’ (P= 0.005; OR = 28.523; 95% CI = 2.683-303.171) was determined to be an independent and significant predictor of improvement in visual field defects caused by intracranial aneurysms (Table 4).
Discussion
Anatomical consideration
Aneurysms that cause visual deficits due to anterior optic pathway compression can arise in any portion of the circle of Willis [10,11,13,14,18-20]. However, aneurysms that arise from the ICA, especially the paraclinoid ICA, are the most common cause of visual deficits caused by aneurysmal compression of the anterior optic pathway [10,14]. Here, the ophthalmic segment was the most common location for UIAs that cause visual deficits. Because the ophthalmic segment of the ICA is located just below the optic nerve and optic chiasm, UIAs that arise from this location may induce mass effects on the optic pathway. In addition, these UIAs can also displace the optic nerve toward bony structures and thereby further compress the optic nerve. These direct compression mechanisms are believed to be the main cause of visual deficits. On the other hand, the blood supply to the anterior optic pathway can be diminished by a kinked ophthalmic artery, and blood flow through the small arterial branches in the parasellar or suprasellar regions can be reduced by the mass effects of the UIAs [10,14]. The International Study of Unruptured Intracranial Aneurysms Investigators reported that the sizes of almost all UIAs are less than 10 mm [1], but 88.5% of UIAs that caused visual deficits were larger than 10 mm in our study and other studies reported similar results [10-13]. Large and giant aneurysms that arise from the cavernous segment are well-known causes of 3rd, 4th, 5th, and 6th cranial nerve dysfunction [10,21,22]. However, our current study and others report that giant aneurysms that arise from the cavernous segment can cause visual deficits [10,23,24].
Choice of treatment modality
Several previous studies have reported that endosaccular coiling is as effective as surgical clipping for treating 3rd nerve dysfunction due to posterior communicating artery aneurysms, and reducing aneurysmal pulsatility without removing mass effects may be an important mechanism for recovering 3rd nerve function [2,25-28]. Therefore, our current study also has placed emphasize on whether endosaccular coiling is as effective as surgical clipping for the treatment of visual deficits caused by aneurysms that arise from the ICA and has further focused on the mass effects of endosaccular coiling. According to our present results, patients in group A, who achieved not only the removal of the mass effects of the aneurysm but also reduced aneurysmal pulsatility, demonstrated visual improvement significantly more often in comparison with group B patients who achieved only the reduction of aneurysmal pulsatility. Aneurysm size, symptom duration, intra-aneurysmal thrombosis, involved quadrants in the visual field, and involved location in the anterior optic pathway were not predictive of favorable outcomes. Only the treatment modality ‘group A’ (P= 0.005; OR = 28.523; 95% CI = 2.683-303.171) was determined to be an independent predictor of improvement in visual field defects caused by intracranial aneurysms that arise from the ICA. Schuss et al. [17] also reported that 75% of patients who were surgically treated achieved improvement in visual symptoms in comparison with 38% of patients who received endocascular treatment. In addition, other studies reported that the only 36.8% and 50% of patients who presented with visual deficits due to intracranial aneurysms achieved improvement following endosaccular coiling [13,29,30]. Therefore, we here report that it is very important to remove both aneurysmal pulsatility and mass effect in the anterior optic pathway in order to improve visual deficits.
Flow-diverting devices were recently introduced as an alternative modality for treating intracranial aneurysm [31-33]. Flow-diverting devices facilitate endoluminal reconstruction of the parent artery and lead to blood flow stagnation and thrombosis in the aneurysm [34-36]. These devices gradually reduce aneurysmal pulsatility and mass effect. Several studies have reported that 90%-100% of patients who had symptomatic intracranial aneurysms either completely or partially recovered from their symptoms after treatment with flow-diverting devices [34,37,38]. However, the complete occlusion rate of the flow-diverting devices for the treatment of giant ICA aneurysms is still lower than the microsurgical techniques [35,39,40]. In addition, the delayed ruptures of large or giant aneurysms after treatment with flow-diversion devices have been reported [41,42]. Nevertheless, flow-diverting devices may be the alternative for the treatment of symptomatic ICA aneurysm because of lower morbidity and mortality rates than traditional microsurgery, including clipping and bypass and avoiding the mass effect associated with coiling [35,39,40,43-45].
Visual deterioration after endosaccular coiling
Visual symptoms are aggravated in 4 patients in group B (40%) after endosaccular coiling. It has been reported that visual deterioration after endosaccular coiling might be caused by thromboembolic events including retinal infarction and occipital lobe infarction, which have an abrupt and maximal onset [13,46]. However, we did not identify the presence of retina infarction and there was no patient who experienced occipital lobe infarction after treatment in present study. Progressive worsening of vision, which occurs hours to weeks after the procedure, may be secondary to progressive mass effect from aneurysm thrombosis and/or perianeurysmal inflammation and edema [46,47]. Turner et al. [46] reported that HydroCoil (MicroVention, Aliso Veijo, California, USA) component of the coil mass could be contributing to perianeurysmal edema and inflammation. HydroCoil is usually commonly used in our institution. Because the 4 patient experienced progressive worsening of vision, which occurred several hours after endosaccular coiling, we speculate that the causes of visual deterioration were aneurysm thrombosis and/or perianeurysmal inflammation and edema. However, these visual deteriorations did not improve satisfactorily after administration of corticosteroids.
Microsurgical strategies
The surgical exposure and dissection of an aneurysm from the anterior optic pathway that are required to perform direct clipping are difficult procedures. Sometimes, visual deficits worsen after the direct clipping of aneurysms that arise from the ICA due to surgical injury. There are 2 patients who experienced aggravated visual symptom after surgical clipping in present study. It has been reported that postoperative vision deterioration might be caused by thermal injury to the optic nerve during drilling of the bony structures, contusion of the anterior optic pathway during dissection and traction, compromised blood flow through the ophthalmic artery, and retinal ischemia due to temporary occlusion [11,13,48,49]. Therefore, we used several surgical techniques to reduce the deterioration of visual function. First, we used extradural anterior clinoidectomy rather than intradural anterior clinoidectomy. Extradural anterior clinoidectomy demonstrates several advantages over intradural anterior clinoidectomy: 1) anatomical orientation can be easily identified by dural extension into the optic canal and superior orbital fissure; 2) extradural clinoidectomy can be performed faster than intradural procedure; and 3) the dura protects the intradural structure including the optic nerve from thermal and mechanical injury [50,51]. Second, we use intraoperative indocyanine green angiography after clipping. Indocyanine green angiography demonstrates several advantages, including good spatial resolution, simple dye injection, rapid performance, and the ability to morphologically and dynamically verify blood flow [52]. Hence, distal flow of the ophthalmic artery can be easily verified using indocyanine green angiography during microsurgery. Third, we used the retrograde suction decompression technique when necessary, as described in previously published studies [53,54]. Proximal control of large and giant aneurysms that arise from the ICA is very difficult because of their relationships with bony structures, adjacent brain structures, anterior choroidal artery, ophthalmic artery, small perforators, and optic nerves [54,55]. Furthermore, even after proximally controlling the parent artery, aneurysms can still remain tensive due to their size and collateral supply [55]. The retrograde suction decompression technique not only easily achieves proximal control at the cervical ICA, but can also deflate aneurysms. Hence, the retrograde suction decompression technique is useful for preventing injury to the optic nerve and ophthalmic artery.
If a large or giant aneurysm has a calcified wall, intra-aneurysmal thrombus, or if some portion of the aneurysm is located in the cavernous segment of the ICA, it may not be possible to treat the aneurysm with direct clipping. Endovascular parent artery trapping without endosaccular coiling can be a good alternative in these situations. However, ischemia-related morbidity and mortality have been reported after occlusion of the ICA [56,57]. Therefore, we use balloon occlusion test before ICA trapping to identify patients at high risk of ischemic complications following ICA sacrifice. If a patient does not pass balloon test occlusion, we perform extracranial-intracranial bypass before parent artery trapping. Performing endovascular parent artery trapping on large or giant aneurysms that arise from the ICA is one of the most useful treatments because this procedure does not manipulate the optic nerve and the ophthalmic artery, and this procedure does not result in additional masses in the anterior optic pathway after treatment.
Limitations
There are several major limitations to this study. First, this study is limited by the small number of cases and its retrospective study design. Second, owing to the different skill sets and preferences of the three members of our cerebrovascular team (two neurosurgeons and one neuro-interventionist), there is inherent selection bias. Naturally, clinicians tent toward methods at which they are highly skilled. Another important limitation of this study is that the period, duration, and modality of follow-up after treatment were not standardized because of the diversity of patient conditions, and the different treatment modalities. In addition, the mean follow-up duration for visual field examination is relatively short.
Conclusion
Visual deficits caused by anterior optic pathway compression due to ICA aneurysms can improve after surgical clipping, endovascular parent artery trapping, and endosaccular coiling. However, based on our experiences, the treatment modality is the only predictor of improvement in visual deficits. In our present study, treatment without endosaccular coiling, including surgical clipping and endovascular trapping with or without bypass surgery, achieved visual improvement significantly more often in comparison with endosaccular coiling. Based on our experiences and literature reviews, we believe that removing aneurysmal mass effects in the anterior optic pathway demonstrates a major impact on improving visual deficits.
Notes
The authors have no financial conflicts of interest.