Impact of Provoking Risk Factors on the Prognosis of Cerebral Venous Thrombosis in Korean Patients
Article information
Abstract
Background and Purpose
Little is known about the relationships between provoking risk factors, prognosis, and optimal duration of anticoagulation in patients with cerebral venous thrombosis (CVT), especially in Asians. We aimed to investigate whether the prognosis and required duration of anticoagulation in CVT patients differ according to the provoking risk factors.
Methods
Prospectively recorded data from a tertiary medical center in South Korea were retrospectively reviewed. CVTs were categorized into three groups: unprovoked, those with possibly resolved provoking factors (PR), and those with persistent provoking factors (PP). The baseline characteristics, treatment, and prognosis of patients in these three groups were analyzed.
Results
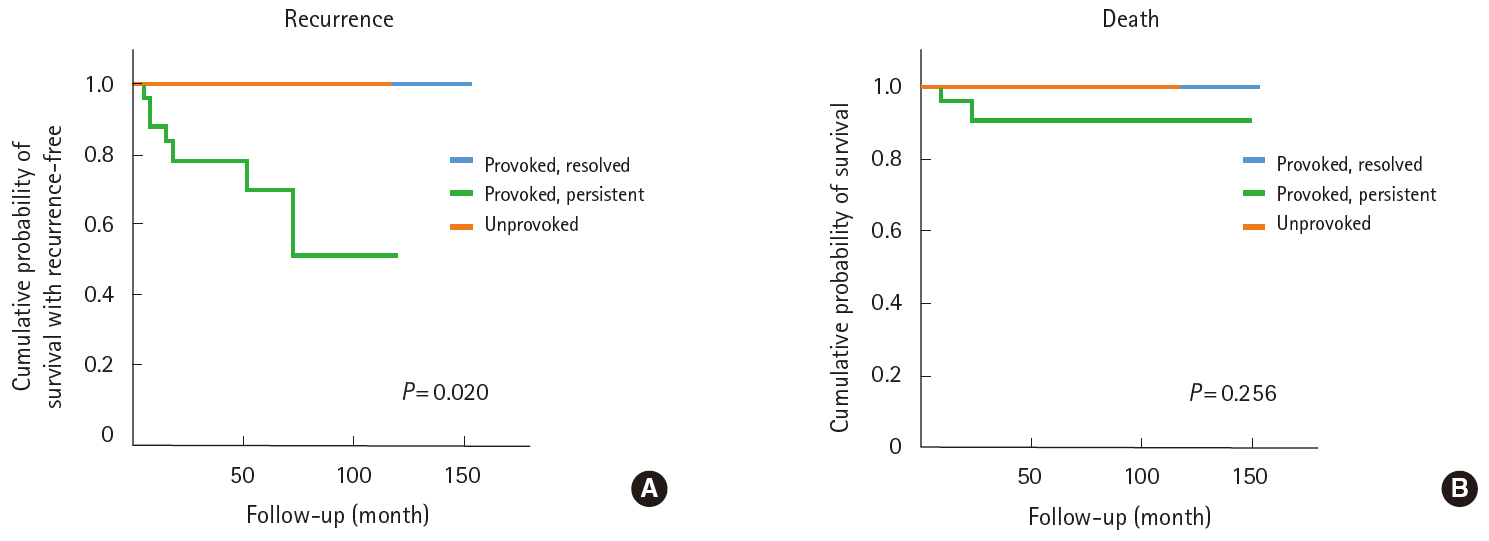
From 2000 to 2015, 61 patients presented with CVT: 19 (31.1%) unprovoked, 11 (18.0%) with PR, and 31 (50.9%) with PP. The patients in our cohort had a slight female predominance and lower frequency of oral contraceptive use compared to Western cohorts. Median follow-up and duration of anticoagulation were 35 and 8 months, respectively. Despite the similarities in baseline characteristics, deaths (n=3; P=0.256) and recurrences (n=7; P=0.020) were observed only in the PP group. The median intervals to death and recurrence were 9 and 13 months, respectively. Death was associated with underlying disease activity, not with CVT progression. Recurrences in the PP group were associated with lack of anticoagulation (P=0.012).
Conclusions
Although the prognosis of CVT is generally benign in Koreans, recurrence and death were observed in patients with persistent risk factors, suggesting their need for long-term treatment with anticoagulants.
Introduction
Cerebral vein and dural sinus thrombosis (CVT) is a rare disease, which most often affects young adults [1-4]. Its prognosis is generally benign [5-9], although recurrence and death during follow-up have been observed [2]. Various risk factors for CVT have been identified [9-21], some of which may resolve during follow-up. Despite recent controversies [22,23], anticoagulant treatment is recommended to reduce morbidity and mortality [3-5,24]. As long-term anticoagulation often provokes bleeding complications, determining the optimal duration of therapy is important.
The duration of anticoagulation in CVT patients may be determined by the provoking risk factors, as is the case for venous thromboembolism [3]. Patients with a first episode of venous thromboembolism related to a reversible risk factor are strongly recommended to receive a short course of anticoagulation, while it is recommended that patients with unknown or recurrent risk factors are maintained on anticoagulation for months to years, or even indefinitely [25,26]. However, such a strategy of determining duration of anticoagulation relative to causative factors has not been firmly established in patients with CVT [27].
Lately, data from Western countries, reflected in a recent guideline, have suggested that indefinite anticoagulation may be considered in CVT patients with severe thrombophilia, defined by congenital thrombophilia; deficiencies of antithrombin, protein C or protein S; and presence of antiphospholipid antibodies [3-5]. However, whether such long-term treatment is also necessary for patients with other persistent provoking factors is unknown. In addition, the same strategy may not be directly applicable to Asian patients. The long-term prognosis of CVT has not been established in Asian patients; moreover, the risk factors may be distinct. For example, factor V Leiden and prothrombin G20210A mutations, which constitute a major proportion of severe thrombophilia, are extremely rare in Asians [28].
To provide more information on the prognosis of CVT in Asian patients, this study aimed to analyze treatment data and long-term prognosis in Korean patients with CVT classified by the presence and type of provoking risk factors. We considered that the relationships of causative risk factors and treatment status with the long-term prognosis of patients with CVT may be useful in assessing the need and duration of anticoagulation.
Methods
Patients
This study retrospectively evaluated prospectively registered, consecutive patients aged >20 years who were diagnosed with symptomatic CVT at the Department of Neurology, Asan Medical Center, Seoul, Korea, between June 2000 and February 2015. In our center, both patients with ischemic stroke and those with intracranial hemorrhage, who are not in need of emergency neurosurgery, are usually admitted to the neurology intensive care unit or to the stroke unit located in the neurological ward, and are managed by neurologists. A diagnosis of CVT was considered when a patient complained of severe headache, seizure, or any stroke-like symptoms, with a venous sinus or cortical vein thrombosis shown on imaging work-up, often combined with parenchymal lesions of unusual vascular territories. Only patients who had this confirmed by conventional angiography, CT and/or MR venography were diagnosed with CVT and included in the analysis. Patients were followed up from diagnosis until June 2015. The study protocol was approved by the Institutional Review Board and the Ethical Standards Committee of Asan Medical Center.
Clinical and laboratory data and grouping
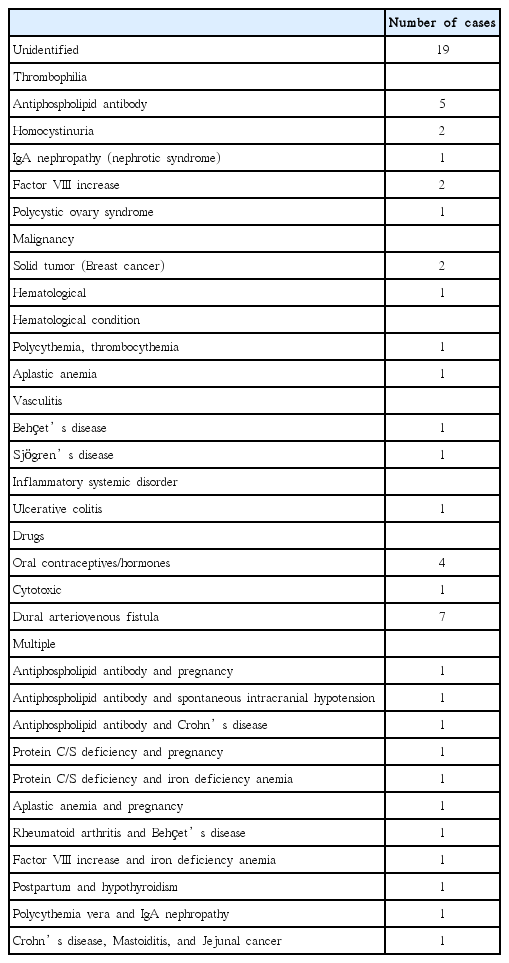
Clinical and laboratory data at the time of the first event were recorded, as were recurrences and deaths. Potential risk factors were those suggested by previous studies [1,9] (Table 1).
Patients suspected of having CVT underwent laboratory tests for coagulation and thrombophilia, including functional and/or antigenic assays for D-dimer, antithrombin-III, proteins C and S, anti-phospholipid antibodies, and coagulation factors; more than 85% of patients had all of these tests. C-reactive protein levels and erythrocyte sedimentation rate were also assessed. As anticoagulants may affect measurements of coagulation factors and proteins C and S, patients were diagnosed with factor VIII increase or protein C/S deficiency only when the blood samples were obtained before the initiation of anticoagulants or when the abnormalities were repeatedly observed after the discontinuation of anticoagulants. As factor V Leiden and prothrombin G20210A mutations are rare in Asians [28], genetic tests for congenital thrombophilia are not performed in our institution.
CVT provoking risk factors were classified into two categories: those provoked, resolved (PR) after the first event and those found to be provoked, persistent (PP). The patients were categorized into three groups: unprovoked, PR, and PP.
Treatment, follow-up, recurrences, and deaths
Although most CVT patients were treated initially with anticoagulants, some patients with mild symptoms, stent insertion, severe thrombocytopenia with bleeding, or successful arteriovenous fistula (AVF) embolization, were given antiplatelets or were not treated. Patients with severe sinus thrombosis underwent fibrinolysis and stent angioplasty at the discretion of the attending physicians. Patients were followed up for recurrence and death in the outpatient clinic every 3-6 months. Recurrence was defined as newly developed symptoms, accompanied by new parenchymal lesions, or progression in sinus thrombosis on imaging work-ups. Death from any cause was recorded, as were major bleeding complications (i.e., intracranial hemorrhage, hemorrhage of any cause, and a ≥3 g/dL reduction in hemoglobin concentration from baseline).
Statistical analysis
Baseline characteristics and laboratory data were compared in the unprovoked, PR, and PP groups, and in PP patients with and without recurrences. Univariate analysis was performed using Fisher’s exact test, Kruskal-Wallis one-way analysis of variance by ranks, or the Mann-Whitney U test, as appropriate. Recurrence- and death-free survival rates were calculated by the Kaplan-Meier method, and compared by the log-rank test, which tested the null hypothesis, that there was no difference in rates of recurrence and death among the three groups. A two-tailed P value <0.05 was considered statistically significant. All statistical analyses were performed using SPSS (version 21) software package.
Results
Baseline characteristics
The study cohort included 61 patients with CVT. Their median age was 41 years, and there was a slight female predominance (55.7%). Of the patients, 45 (73.8%) underwent conventional angiography. Median follow-up and duration of anticoagulation were 35 and 8 months, respectively. Provoking risk factors were identified in 42 patients (Figure 1 and Table 1). Seventeen (27.9%) had thrombophilia with some of these patients having multiple risk factors, and only four out of 34 (11.8%) female patients had used oral contraceptives. Malignancy was identified in four patients: 2 with breast cancer, 1 with jejunal cancer, and 1 with aplastic anemia. All of those malignancies remained active throughout the study. Among the identified risk factors regarded as PR were previous history of using causative drugs, and AVF successfully treated during admission. Thus, of the 61 patients, 19 (31.1%) were classified as having unprovoked CVT, 11 (18.0%) as having PR, and 31 (50.9%) as having PP.
Comparisons of the three groups
A comparison of baseline characteristics among the three groups showed no significant differences except for duration of anticoagulation, intervention methods, and C-reactive protein (CRP) concentration (Table 2 and Supplementary Table 1). Patients in the PR group tended not to receive anticoagulants (and those who did received them for a shorter duration) and had a lower CRP level than patients in the other groups.
Recurrences and deaths
During the study period, three patients (4.9%) died, and seven patients (11.5%) experienced recurrences, all of whom were in the PP group (Figure 2); overall, thirty-seven (60.7%) patients experienced an event recurrence or dropped out. During follow-up, no patients experienced major bleeding complications, except for one patient with thrombocytopenia (patient 3 in Table 3). The median intervals from the first event to death and to recurrence were 9 and 13 months, respectively.
Of the three patients who died, two died from aggravation of the underlying disease: cancer in one patient, resulting in severe pneumonia; and aplastic anemia with thrombocytopenia in the other, resulting in massive intracranial hemorrhage. The third death was in a patient with antiphospholipid antibody who developed a sudden cardiac arrhythmia.
The rates of recurrence in the unprovoked, PR, and PP groups differed significantly (Figure 2A). A comparison of the baseline characteristics of PP patients with and without recurrence showed that use of anticoagulation was the factor that differed significantly (Table 4).
Patients with recurrences
The characteristics of each CVT patient who developed recurrences are described in Table 3. After the first event, all patients showed improvement in their symptoms at discharge, or in lesions and/or thrombosis on follow-up images (if available). Although four patients were discharged with anticoagulants after the first event, only two were receiving anticoagulation at the time of recurrence. Notably, four patients developed recurrences more than 12 months after the first event. Patient 6 with dural AVF, who failed with embolization at the initial admission due to severe collapse of sinuses, was regarded as having PP. At the second event, he underwent interventional treatment of the dural AVF again, consisting of subtotal embolization of the AVF and stent angioplasty in the transverse sinus. This time, the treatment was successful, and no recurrences were observed thereafter. Of the six other patients, five experienced no recurrences after the restart of anticoagulation. Patient 3 was exceptional, because she could not receive sufficient anticoagulation continuously during follow-up due to recurrent menorrhagia induced by thrombocytopenia. She later died after a massive intracranial hemorrhage.
Discussion
In this study, we identified CVT risk factors in a Korean cohort, and evaluated the long-term prognosis of the disease according to the presence and type of provoking factors. We found that although the prognosis of CVT is generally benign in Koreans, risk factors persisting during follow-up should be considered significant for secondary prevention. These findings also suggest that patients with persistent risk factors may require long-term anticoagulation.
Among the three groups defined by risk factors, only those with PP experienced recurrences and deaths. The persistent risk factors associated with recurrence in our cohort varied, and were not limited to severe thrombophilia [4,5]. The causes of death in the three patients who died were not directly related to the aggravation of CVT, but to the activity of underlying diseases, in agreement with previous findings [9,17,22].
Appropriate anticoagulation was shown to be important, as anticoagulation was less frequent in patients with recurrences than those without. The median interval from the first event to recurrence was 13 (range: 3-71) months, suggesting that patients with persistent risk factors may require anticoagulation for several years or more. These findings are reminiscent of some previous studies arguing that anticoagulation is important in preventing recurrences in the long-term [5,18]. Of note, other research groups have reported that warfarin therapy has no effect on recurrent thrombosis or survival, although those studies did not describe the prothrombin time in patients who developed recurrence while on warfarin [22,23]. Thus, our findings provide further evidence for the importance of long-term anticoagulation in the secondary prevention of CVT in patients with persistent risk factors.
The risk factors provoking CVT in our cohort were distinct, in that the percentage of patients with a history of oral contraceptive use was relatively small compared with previous studies (54.3%-90%) [5,9,17,22]. This discrepancy may be due to the lower rate of use of oral contraceptives in East Asia (1.3%) than in Europe (21.4%) and North America (16.8%) [29]. Less frequent use of oral contraceptives, usually a transient risk factor unrelated to recurrence, may have resulted in a relatively low proportion of female patients and a high rate of recurrence (11.5%) in our patients. Thrombophilias (27.9%) were found in a rate similar to those in other studies (24.0%-38.1%), although we did not perform genetic tests to identify congenital thrombophilia. As congenital thrombophilia is very rare in Asians [28], the absence of genetic tests may have not significantly altered the risk factors identified in this population.
The PR group consisted of patients with dural AVF who were successfully treated with embolization, and those with CVT caused by drugs. Dural AVF, characterized by a shunt between the artery and sinus, can induce turbulent flow into the venous sinus that causes intimal injury leading to luminal thrombosis [18,30]. In addition, secondary CVT may amplify venous hypertension, which induces retrograde flow into the sinus [30]. Dural AVF can also develop as a late complication of CVT [4]. However, the possibility of this occurring was not likely in our cohort, since the median delay in diagnosis of CVT from symptom onset in these patients was only 6 days. Five patients were found to have causative drugs, including four with a history of oral contraceptive use and one taking cisplatin and etoposide for a pineal gland germinoma. All of these patients ceased using these agents after the first event. The patient with a history of chemotherapy was classified in the PR group, as pineal germinoma has not been associated with thrombosis, and this patient was successfully treated with radiotherapy after the discontinuation of the chemotherapeutic agents. Pregnancy and puerperium can also be transient risk factors. However, all of our patients with a history of pregnancy or puerperium also had other, persistent risk factors, classifying them in the PP group.
This study had several limitations. Firstly, because of its retrospective design, the patients did not all undergo the same laboratory tests to identify risk factors. Thus, some of the patients classified in the “unprovoked” group may have had provoking risk factors. Inasmuch as recurrences and deaths were observed only in CVT patients classified as PP, the non-identification of risk factors in unprovoked patients would likely not have altered the results of this study significantly. Secondly, we did not consider or investigate venous thromboembolism events during follow-up, despite venous thromboembolism being a more frequent complication of CVT than CVT recurrence [5]. Third, there is a chance that we did not include fulminant cases in patients who manifested with severe intracranial hemorrhage requiring emergency neurosurgery, as they would have been admitted to the department of neurosurgery directly from Emergency Room. Not including these patients may have affected the long-term prognosis of CVT, shown in this study to appear relatively benign. Fourth, this study involved patients from a single center, suggesting a potential referral bias. As CVT is a rare disease, studies of a large cohort of patients from multiple centers may provide more reliable results.
Conclusion
This study showed that the long-term prognosis of patients with CVT is dependent on underlying risk factors, although the natural course of this disease in Koreans is generally benign. CVT recurrence and death were observed only in patients with persistent risk factors, with some occurring more than one year after the first event whilst on inadequate anticoagulation. Thus, these patients may require long-term treatment with anticoagulants.
Notes
This study was supported by a grant of the Korea Healthcare technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI10C2020).
The authors have no financial conflicts of interest.
Supplementary Material
Initial symptoms, affected sinuses, and the location of parenchymal lesions