Adult Stem Cell Therapy for Stroke: Challenges and Progress
Article information
Abstract
Stroke is one of the leading causes of death and physical disability among adults. It has been 15 years since clinical trials of stem cell therapy in patients with stroke have been conducted using adult stem cells like mesenchymal stem cells and bone marrow mononuclear cells. Results of randomized controlled trials showed that adult stem cell therapy was safe but its efficacy was modest, underscoring the need for new stem cell therapy strategies. The primary limitations of current stem cell therapies include (a) the limited source of engraftable stem cells, (b) the presence of optimal time window for stem cell therapies, (c) inherited limitation of stem cells in terms of growth, trophic support, and differentiation potential, and (d) possible transplanted cell-mediated adverse effects, such as tumor formation. Here, we discuss recent advances that overcome these hurdles in adult stem cell therapy for stroke.
Introduction
Stroke is the leading cause of physical disability among adults. One-fourth to a half of stroke survivors are left with significant disabilities. Stem cell therapy is considered a potential regenerative strategy for patients with neurologic deficits. This review presents the most recent advances in adult stem cell therapy for stroke, focusing on improvements in its safety and efficacy profile.
Search strategy and selection criteria
We identified references for this review by searching PubMed and ClinicalTrials.gov published in English up to December 2015, with the search terms stroke, cerebral infarction, stem cells, and mesenchymal stem cells (MSCs). Additionally, we searched references from relevant articles and reviews. The final reference list was generated on the basis of originality and relevance to this topic. Because of space limitation, we are not able to discuss many potential regenerative strategies of adult stem cells therapies in depth with critical analysis. Genetically modified stem cells are mentioned only briefly because they have some limitations in the clinical use in stroke patients.
Mechanisms of action of adult stem cells in stroke
A critical limitation of adult stem cell therapy for stroke is the lack of complete understanding of mechanism of action mediating the observed therapeutic benefits. Embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) can replace the missing brain cells in the infarcted area. However, there are hurdles associated with true neuronal substitution using cell replacement to restore neuronal function after stroke [1], and ESC/iPSC therapy was associated with tumor formation. Moreover, preclinical studies showed that most transplanted cells disappeared within a few weeks [2]. In fact, a low graft persistence of transplanted cells could be a positive readout for the safety of stem cell therapies (i.e., unregulated stem cell proliferation).
Therefore, adult stem cells such as MSCs may be a good choice for stroke therapy because they secrete a variety of bioactive substances, including trophic factors and extracellular vesicles (EVs, 0.1–1 μm sized circular membrane fragments shed from the cell surface), into the injured brain, which may be associated with enhanced neurogenesis, angiogenesis, and synaptogenesis [3-7]. In addition, MSCs are thought to play multiple roles, such as attenuating inflammation [8,9], reducing scar thickness [10], enhancing autophagy [11], normalizing microenvironmental/metabolic profiles [12], and possibly replacing damaged cells [13] in various brain diseases.
Results of clinical trials
With current advances in understanding the effects of introducing stem cells and their mechanisms of action, several clinical trials of stem cell therapy were conducted in patients with stroke. Most clinical trials used adult stem cells, such as MSCs [14-18] and bone marrow mononuclear cells [19-24]. While stem cells appeared to be of some benefit in several studies, there was significant bias in subsequent studies [25].
In the STARTING (STem cell Application Research and Trials In NeuroloGy) trial, autologous MSCs culture-expanded with fetal bovine serum were applied intravenously in the subacute phase of stroke [15]. Intravenous autologous MSC transplantation was safe for stroke patients through a long-term period, and may improve recovery. However, many patients in the MSC group remained significantly disabled [15]. More recently, the InveST (Intravenous Autologous Bone Marrow Mononuclear Cell Therapy for Ischemic Stroke) trial tested whether intravenous infusion of autologous bone marrow mononuclear cells between 7 to 30 days after stroke onset could result in a reduction of the infarct volume and could improve neurological function at day 180 of follow-up [24]. This trial failed to show effectiveness.
Because these randomized, observer-blinded trials were not pivotal efficacy trials, there can be no conclusions made regarding the efficacy of this therapy. Some of the failures in clinical trials may be predicted on the basis that there was insufficient preclinical data to support a strong clinical benefit [26]. Improvement in the therapeutic efficacy of current stem cell therapies is needed before efficacy studies are conducted. Very recently, the results of a phase II, multicenter, double blind randomized controlled trial of intravenous application of allogeneic multipotent adult stem cells (the B01-02 trial of MultiStem®) in acute stroke patients have been reported [18]. MultiStem®, an allogeneic “off-the-shelf” cell therapy, during acute phase of stroke (within the 24-48 hour window) was safe and significantly improved recovery and reduced mortality at one year.
Hurdles of current adult stem cell therapies
The primary hurdles for current stem cell therapies include (a) the limited source of engraftable stem cells, (b) the presence of optimal time window for stem cell therapies, (c) inherited limitation of adult stem cell potential, and (d) possible transplanted cell-mediated adverse effects, such as tumor formation or stroke (Figure 1).

Hurdles of current stem cell therapy and strategies to overcome the challenges. MSC, mesenchymal stem cell; iPSC, induced pluripotent stem cells; ESC, embryonic stem cells; BBB, blood-brain barrier; EV, extracellular vesicle.
First, although the optimal cell dosage and route of administration of stem cells in cardiovascular diseases are not settled [27], administration of sufficient cell dose is mandatory to have beneficial effects of stem cells. Unlike to hematogenous stem cells, the number of MSCs in bone marrow dramatically decline with age, requiring culture-expansion [28]. It was reported that MSCs enter senescence and are losing their stem cell characteristics (plasticity and homing ability) during ex vivo culturing [4,29,30]. In addition, stroke mostly occurs in elderly people, and MSCs obtained from elderly patients show the decline in proliferation, self-renewal, or differentiation capacity.
Second, the optimal time point for the application of stem cells exists, in terms of stem cell tropism toward brain and mechanistic targets of stem cells. The levels of chemokines, trophic factors, and relevant microRNAs (miRs) increased markedly in the infarcted brain during the acute phase of stroke, but decreased with time [31]. In addition, the mechanistic targets for cell therapy may differ depending on temporal windows after stroke. The application of stem cells during acute phase of stroke may be needed to have a range of paracrine and immunomodulatory effects, which lead to a reduction in secondary injury processes and stimulation of brain repair after stroke [32].
Third, adult stem cells may have inherited limitations. MSCs are heterogenous and contain many different types of progenitor or stem cells, in terms of growth, trophic support, and differentiation potentials. The neurorestorative potential of MSCs may be limited in the elderly who have a limited number of neural stem/progenitor cells (NSCs) [33] and bone marrow MSCs [28], who are unable to receive rehabilitation therapy [34], and those with extensive damage to the subventricular region [15]. An attenuation of the regenerative potential of stem cells in aged patients with stroke could result from aging in either the donor cells (e.g., bone marrow stem cells) or the recipient cells (e.g., NSCs in the innate neurogenesis system of the brain). However, stroke-induced neurogenesis has been observed in stroke patients in their 60s and 70s [35]. Although the number of NSCs decreased with age in the human brain [33] and basal neurogenesis was impaired in the subgranular and subventricular zone of aged animals, the degree of neurogenesis after stroke was similar in young and old animals [36]. In addition, NSCs in aged brains could be activated by application of “younger” stem cells. One recent study showed that secreted factors from the young stem cell niche rescued the numbers of NSC colonies derived from old-age subependyma, and enhanced NSC proliferation in vivo in aged animals [37]. On the contrary, age-related changes could affect certain biological features of bone marrow MSCs, resulting in decreased proliferation and paracrine functions as well as increased senescence and apoptosis, which may decrease the neurogenic potential of MSCs [38-41]. These findings suggest the importance of the aging/rejuvenation of donor cells to the neurogenic potential of stem cell therapy.
In addition, the discrepancy in stem cell effects between preclinical and clinical studies may be in part derived from differences in the regenerative potential of healthy young animals and aged patients with chronic disease. One study showed that treatment with bone marrow MSCs in type I diabetic rats increased mortality and blood-brain barrier (BBB) leakage, resulting in brain hemorrhage, and underscored the possibility that stem cell therapy may not be beneficial for diabetic subjects with stroke [42]. Preclinical and clinical studies have also shown that the proliferation and angiogenic capacity of endothelial progenitor cells and MSCs were impaired in patients with coronary artery disease and metabolic disorders [43]. Therefore, further studies are required examining the effects of stem cell therapies for stroke in aged animals with chronic diseases.
Lastly, a major concern with stem cell therapy is cell-mediated adverse effects, i.e., tumor formation of transplanted cells (i.e., iPSC or ESC) that may delay the recovery after stroke [44] and trapping of stem cells in the lung (intravenous application) or brain vessels (intra-arterial application) [45,46].
Recent advances in stem cell research for stroke therapy
The following issues will be addressed in order to overcome these hurdles (Figure 1).
Differential sources of stem cells
Although bone marrow-derived MSCs are most commonly used, other sources of MSCs, such as adipose tissue or the umbilical cord, are increasingly utilized in clinical practice. This has resulted from our current understanding of the characteristics of different sources of MSCs [47-50]. These other sources of MSCs outside bone marrow or allogeneic MSCs from young donors may address the apparent decrease in the number and function of bone marrow MSCs in aged persons. Both adipose tissue and umbilical cord are promising sources of MSCs. Adipose tissue-derived stem cells (ADSCs) are reported to have several advantages over bone marrow-derived stem cells. Relatively large number of ADSCs can be separated from subcutaneous fat tissue with minimally invasive procedures. ADSCs were reportedly superior to bone marrow MSCs in their paracrine functions [51] and angiogenic potential [52]. Moreover, ADSCs were more refractory to the effects of advanced donor age in a mouse model of stroke [47]. MSCs can also be obtained from umbilical cord blood and Wharton’s jelly, etc. Umbilical cord MSCs expressed preferentially secreted factors related to neuroprotection, neurogenesis, and angiogenesis [49,50], Umbilical cord MSCs also showed favorable differentiation capabilities and low immunity. Both ADSCs and umbilical cord MSCs reportedly improved functional recovery in animal models of stroke even when administered after a delayed time [48,53].
In addition, allogeneic stem cells could represent a suitable alternative to autologous stem cells. The safety of allogeneic MSC therapy may be closely related to their short-term existence in the host after the application. Allogeneic stem cells have advantages over autologous stem cells. Allogeneic MSCs are scalable from a manufacturing perspective, with standardized procedures. MSCs from younger healthy donors or iPSC- or ESC-derived adult stem cells may differ in terms of their proliferation and neurorestorative capacity, from those of cells obtained from elderly patients with chronic illness. The use of allogeneic MSCs reduces the time required to obtain a sufficient number of cells (the “off the shelf” approach). In the recent clinical trials of intravenous application of allogeneic stem cells (MultiStem®) in patients with acute stroke, stem cells were applied within the 24-48 hours after the onset of symptoms [18]. Post-hoc analysis showed that early treatment (≤rl hour) was associated with a greater benefit at one year of treatment.
However, conflicting results exist. After contact with serum, allogeneic MSCs can be injured by complement, and the viability of allogeneic MSCs after infusion is greatly reduced compared with autologous MSCs [54]. Several approaches based on biomaterials or mimicry of antigen-specific peripheral tolerance have been suggested to reduce the high risk for rejection of allogeneic cells [55]. Moreover, treatment of stroke with bone marrow stem cells obtained from stroke rats resulted in improved functional outcomes compared to treatment with cells from normal rats [56].
Mode of application of stem cells
The mode of application of stem cells may significantly influence the number of cells delivered to target regions as well as the incidence of adverse effects. A major problem in introducing stem cells systemically is that cells may become trapped within organs that filter the blood (first-pass effect). To avoid this, strategies to minimize lung adhesion and improve the homing of systemically introduced cells are used, including different routes of administration. For example, unlike in intravenous infusions, in an intra-arterial approach, pulmonary circulation can be bypassed, which results in superior delivery and sustained presence of stem cells in the ischemic brain [46]. However, an arterial approach may cause arterial occlusion, resulting in stroke; hence, this approach was not found to be superior to an intravenous approach for recovery after stroke [46,57]. More recently, several studies reported that intranasal delivery of MSCs improved neurovascular regeneration and functional recovery after stroke [58,59]. Intranasal delivery may provide a simple, non-invasive, and brain-specific mode for cell therapy.
Molecules larger than 400 Da cannot pass through the BBB, which may affect the efficacy of cell therapy in stroke patients. Intravenous co-administration of stem cells and mannitol, an osmotic agent that can regulate the permeability of the BBB, may improve outcomes in stroke patients. One preclinical study showed that BBB manipulation using intravenous mannitol prior to MSC treatment resulted in increased levels of trophic factors in the infracted brain [60]. As mannitol is already widely used in clinical practice, BBB manipulation using mannitol could be considered in future clinical trials [61].
In terms of chemokine expression and BBB opening, chronic treatment could be advantageous with non-systemic routes (i.e., intra-arterial, intranasal, or intracerebral) and BBB manipulation. Few studies have directly compared the efficacy of various routes of delivery of MSCs, and further studies are needed.
Ex vivo manipulation of stem cells and culture medium
Decreased functional activity of stem/progenitor cells might be mediated by telomerase shortening/decreased telomerase activity, disturbances of the cell secretome, and altered interactions with the microenvironment [62]. Several methods of culture expansion that improved the proliferation, survival, and trophic support, and reduced senescence of MSCs have been reported, including hypoxic/ischemic preconditioning, genetic modification of cells, trophic factor pretreatment, isolation and use of functional subpopulations of stem cells, modulation of intracellular signal cascades, and modification of the cell microenvironment.
The choice of medium is of importance for the end result of the stem cell culture. Use of human serum or human platelet lysate instead of xenogenic fetal bovine serum during MSC expansion enabled rapid expansion and rejuvenation of MSCs without adversely affecting immunophenotype [16,63]. Likely, culture expansion of MSCs with serum obtained in the acute phase of stroke increased cell proliferation rates, increased trophic factor release, and decreased senescence via circulating signals from the infarcted brain area [64]. A clinical trial (Clinical trial identifier NCT 01716481) will determine the effectiveness and safety of autologous MSCs that are culture expanded in autologous serum obtained as soon after stroke as possible [61]. In addition, hypoxic conditions (i.e., 0.1–2% O2, conditions similar to bone marrow) were beneficial to MSCs and might stimulate MSCs to exhibit adaptive responses [65-69].
Treatment with trophic factors or chemical agents may alter MSC characteristics. Preclinical studies of various ischemic models showed that ex vivo treatment with trophic factors or chemical agents (valproate and lithium) during MSC cultivation enhanced the migration of MSCs and trophic support in the ischemic brain [45,70,71].
Lastly, genetic modification of MSCs increased the trophic support of MSCs (e.g., brain-derived neurotrophic factor genemodified MSCs), and migration of MSCs to infarcted brain areas (MSCs transfected to overexpress a chemokine receptor) [72-75].
EVs derived from stem cells
Cell therapy using the secretome (trophic factors, cytokines, or chemokines produced through paracrine secretion) or EVs (e.g., microvesicles and exosomes) derived from stem cells could represent a new, clinically feasible, and cell-free paradigm that would avoid cell-related problems such as tumor formation and infarcts caused by vascular occlusion. Stem cells secrete EVs as well as soluble factors (e.g., trophic factors), and stroke in humans triggers the mobilization of MSC-derived EVs [76]. EVs harbor bioactive molecules such as lipids, proteins and miR, and EVs secreted from stem cells carry more complex cargos than other cellular sources [77]. Encapsulation of bioactive molecules in EVs increases their stability and bioavailability and helps crossing the BBB [78,79]. Stem cell EVs play a critical role in the exchange of information between stem cells and damaged cells and alter the behavior of the target cells (Figure 2). In recent studies, microvesicles secreted from MSCs promoted sciatic nerve regeneration in rats [80]. On the contrary, astrocytes release exosomes enriched in heat shock proteins and synapsin I under stress conditions [81]. Along with others, we have shown that intravenous administration of EVs derived from MSC culture media promotes functional recovery and neuro-vascular plasticity after stroke in rats [82,83]. No studies have examined the effects of stem cell-derived EVs in stroke patients, but a phase 1 study of cord blood-derived MSC EVs in diabetes patients is ongoing (Clinical trial identifier NCT 02138331).
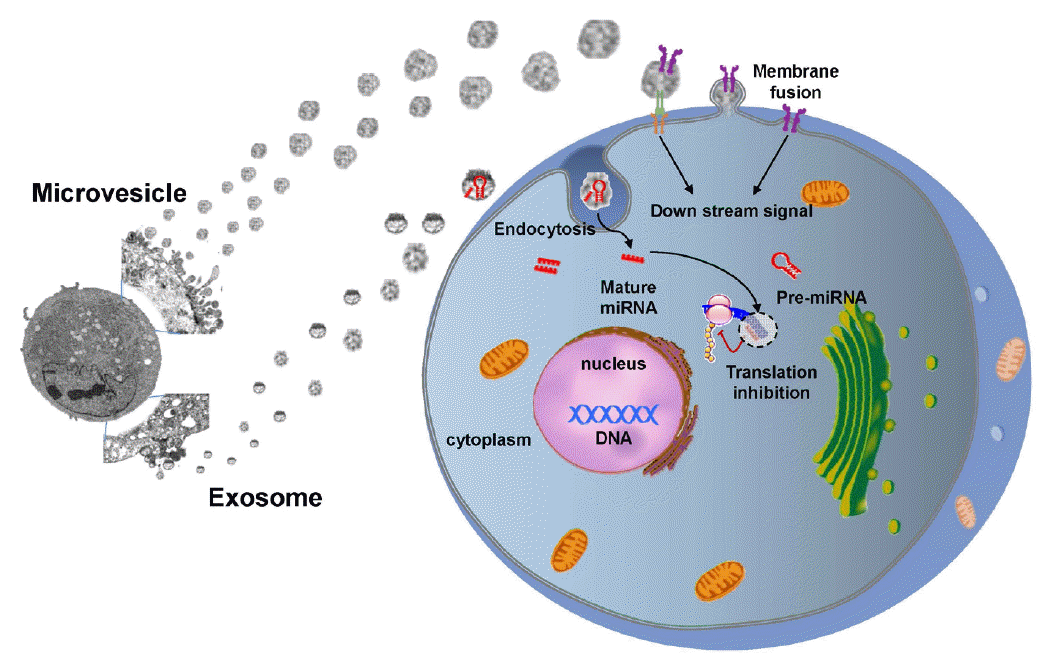
Exchange of information between stem cells and neural cells via EVs. Stem cell-derived EVs can transfer stem cell-specific bioactive molecules, including proteins, mRNAs, and miRs, to injured cells. Thus, EVs can trigger a regenerative program in injured cells in a paracrine manner. Conversely, injured cells may stimulate stem cells by secreting vesicles under pathological conditions.
miRs are short, non-coding RNAs with a capacity to repress varied target genes [84]. Caballero-Garrido et al. investigated an effect of the in vivo inhibition of miR on brain recovery after stroke and showed that intravenous anti-miR-155 injections in a mouse model of stroke improved poststroke angiogenesis and recovery [85]. Extracellular miRs can be protected from degradation by RNase through encapsulation within EVs. Accumulating evidence supports that stem cell-derived EVs help to repair brain damage after stroke via miR delivery to target cells, thereby regulating the expression of genetic information and promoting a therapeutic response [86]. For example, MSC administration increased miR-133b expression levels in rat brain after stroke and exosomal miR-133b transferred from MSCs to neurons and astrocytes regulated neurite outgrowth [87] and neurovascular remodeling to promote stroke recovery [82]. The efficacy of stem cells could be enhanced in principle by regulating miR expression in stem cells or EVs. miR-based therapies, including miR amplifiers and inhibitors, and artificial miRs, are currently being developed [88]. Transplantation of MSCs over-expressing miR-126, promotes functional angiogenesis in the ischemic myocardium [89]. In addition, modification of EV-miRs may occur during the ex vivo manipulation of stem cells, e.g., preconditioning [90,91].
Lastly, administration of small molecules, biomaterials, and biologics that targets the stem cell niche is a promising field in regenerative medicine [92]. Small molecules that modulate specific targets involved in the signaling and mechanisms of stem cells may be used to modulate stem cell function in vitro and in vivo. Compared to cell therapy, small molecules are relatively lower cost, more convenient to use, and offer better temporal/spatial control [93]. However, relatively few studies have been conducted for stroke therapy.
Application of 3D bioprocessing techniques
To date, considerable attention has been focused on the development of novel 3D bioprocesses that can facilitate the clinical translation of stem cell research. Besides the various methods of biochemical and/or genetic preconditioning mentioned previously, the biological properties of stem cells can be manipulated by 3D bioprocessing techniques that physically mimic the natural microenvironments of the stem cells in vivo [94].
It is becoming increasingly apparent that the native phenotype of MSCs is altered with repeated passages during culture expansion using conventional tissue culture flasks [95]. With regard to this, the formation of MSC aggregates can reproduce natural 3D interactions between cells and the extracellular matrix or other neighboring cells, creating an “in-vivo-like” microenvironment where the phenotype and innate properties of the MSCs can be better preserved [96,97]. Current research has shown that MSCs can be easily prepared as spheroid-shaped cellular aggregates by simple 3D bioprocessing techniques, and that these 3D MSC aggregates may have advantages over MSCs from monolayer cultures for many therapeutic applications. For example, secreted anti-inflammatory, proangiogenic, and promitotic factors were highly enriched in 3D MSC aggregates [94,97-100]. Furthermore, the differentiation efficiency of MSCs toward adipogenic, osteogenic, and potentially epithelial-like or neuronal-like phenotypes were also increased by these techniques. Thus, 3D culturing has been proposed as a preconditioning strategy to enhance the therapeutic functions of the MSCs [94,98,99].
Likely, the incorporation with biomaterials can also provide stem cells with a highly biocompatible 3D-environment. Tissue-engineered scaffolds with engineered biochemical/physical properties could support cellular survival in a long-term transplantation to promote integration with the host tissue [101]. Theoretically, the scaffold could serve as a therapeutic cell-delivery vehicle that releases neuroprotective factors in a controlled manner, which would further promote the regeneration and functional revival of damaged neuronal tissue [102]. Recently, an electrically conductive polymer scaffold was developed as a novel NSC delivery system. In this study, human NSCs were electrically preconditioned in 3D by seeding on to the scaffold, which resulted in an improved rate of recovery of neurological functions after stroke [103].
In addition, culture methods to expand 3D bioprocessed stem cells should be further studied. Traditional static culture conditions limit the mass transfer of nutrients, oxygen, and metabolic wastes, resulting in a necrotic center of cellular mass while cells near the outer layer remain viable [104,105]. Therefore, dynamic culture conditions are required to improve the cellular and biomolecular composition of 3D stem cell-based constructs [106-111]. Bioreactor systems can offer not only well-controlled mixing of the culture media but also real-time monitoring of culture parameters. Continuous evaluation of critical culture parameters could facilitate the efficient scale-up of 3D stem cell-based constructs [112,113]. To this end, reproducible, standardized 3D bioprocessing protocols could be established to customize stem cell therapy for stroke. Automated bioreactor systems would allow powerful stem cell therapies to translate to general practice, which might otherwise stay restricted to academic studies or select societal groups.
Conclusions
Clinical data are still being accumulated in patients with stroke. It remains too early to be confident that adult stem cell therapy can improve functional outcomes in patients with stroke. More evidence from randomized trials is needed. In the meantime, continuing efforts are needed at the bench, to develop stem cell strategies with enhanced therapeutic efficacy of stem cells, and at the bedside, through constant dialogues, to meet the Food and Drug Administration’s regulations on stem cell use in clinical applications. The efficacy of stem cell therapy will be improved with advances in our understanding of stem cell biology and with the advances in techniques to modulate stem cell characteristics, including biotechnology and bioengineering (Figure 3).
Notes
This study was supported by the Korea Health Technology R&D Project, the Ministry of Health & Welfare (HI14C16240000).
The authors have no financial conflicts of interest.
