Penumbral Imaging-Based Thrombolysis with Tenecteplase Is Feasible up to 24 Hours after Symptom Onset
Article information
Abstract
Background and Purpose
Thrombolysis >4.5 hours after ischemic stroke onset is unproven. We assessed the feasibility of tenecteplase (TNK) treatment in patients with evidence of an ischemic penumbra 4.5 to 24 hours after onset.
Methods
Acute ischemic stroke patients underwent perfusion computed tomography (CT)/magnetic resonance imaging. Patients with cerebral blood volume (CBV) or diffusion weighted imaging Alberta Stroke Program Early CT Scores (ASPECTS) >6 and mismatch score >2 (defined as >2 ASPECTS regions with delay on mean transit time maps and normal CBV) were eligible for treatment with TNK (0.25 mg/kg). Patients with mismatch patterns enrolled in non-endovascular/non-thrombolysis trials and those without mismatch patterns served as comparators.
Results
The median (interquartile range) baseline National Institutes of Health Stroke Scale (NIHSS) in TNK treated patients (n=16) was 12 (range, 8 to 15). In the untreated mismatch (n=18) and nonmismatch (n=23) groups, the baseline NIHSS was 12 (range, 7 to 12) and 16 (range, 8 to 20; P=0.09) respectively. There was one symptomatic hemorrhage each in the TNK group (parenchymal hematoma [PH] 2) and non-mismatch group (PH 2). Penumbral salvage volumes were higher in TNK treated patients (48.3 mL [range, 24.9 to 80.4]) than the non-mismatch (–90.8 mL [range, –197 to –20]; P<0.0001) patients.
Conclusions
This prospective, non-randomized study supports the feasibility of TNK therapy in patients with evidence of ischemic penumbra 4 to 24 hours after onset.
Introduction
Only 5% to 12% of acute ischemic stroke patients receive intravenous (IV) thrombolysis with tissue plasminogen activator (tPA), due in part to the narrow therapeutic window [1,2]. The efficacy of tPA decreases with time when patients are selected without reference to the state of the ischemic penumbra [3]. Recent phase II studies, using computed tomography perfusion (CTP) and magnetic resonance (MR) derived perfusion weighted imaging (PWI) have assessed IV thrombolysis up to 6 hours after stroke onset [4,5].
Tenecteplase (TNK) is a genetically-engineered variant of tPA that has a longer plasma half-life, higher resistance to plasminogen-activator-inhibitor 1, 14-fold higher fibrin specificity and induces lysis more rapidly compared to tPA [6]. TNK has also been associated with a lower rate of symptomatic hemorrhagic transformation (HT) compared to tPA when administered within 6 hours of symptom onset at a dose range of 0.1 to 0.4 mg/kg [4,7,8]. The feasibility, safety and efficacy of TNK >6 hours after symptom onset is unknown.
We planned an open-label feasibility and safety study of acute treatment with TNK in ischemic stroke patients presenting between 4.5 to 24 hours of symptom onset with penumbral patterns on advanced imaging. We hypothesized that treatment with TNK in those patients with imaging mismatch patterns is associated with reperfusion, early neurological improvement and penumbral tissue salvage.
Methods
Patients
The penumbral-based novel Thrombolytic Therapy in Acute Ischemic Stroke study was a prospective, open-label, non-randomized phase II study (ClinicalTrials.gov, identifier: NCT02101606). Adult acute ischemic stroke patients presenting between 4.5 to 24 hours of symptom onset, or time last known to be well, who underwent CTP or PWI (depending on the availability) were screened. The study protocol was approved by our local Human Research Ethics Committee.
Eligible patients had moderate to severe neurological deficits (National Institutes of Health Stroke Scale [NIHSS] score, 4 to 18); a systolic blood pressure (BP) <180 mm Hg and diastolic BP <105 mm Hg; Alberta Stroke Program Early Computed Tomography scores (ASPECTS) >6 on the initial non-contrast computed tomography (NCCT); CTP mismatch score >2 (defined as >2 ASPECTS regions with evidence of hypoperfusion visible on mean transit time [MTT] and normal cerebral blood volume [CBV]); or PWI mismatch score of >2 (defined as >2 ASPECTS regions with evidence of hypoperfusion visible on MTT maps, but no associated diffusion restriction on diffusion weighted imaging [DWI]) (Figure 1) [9,10].
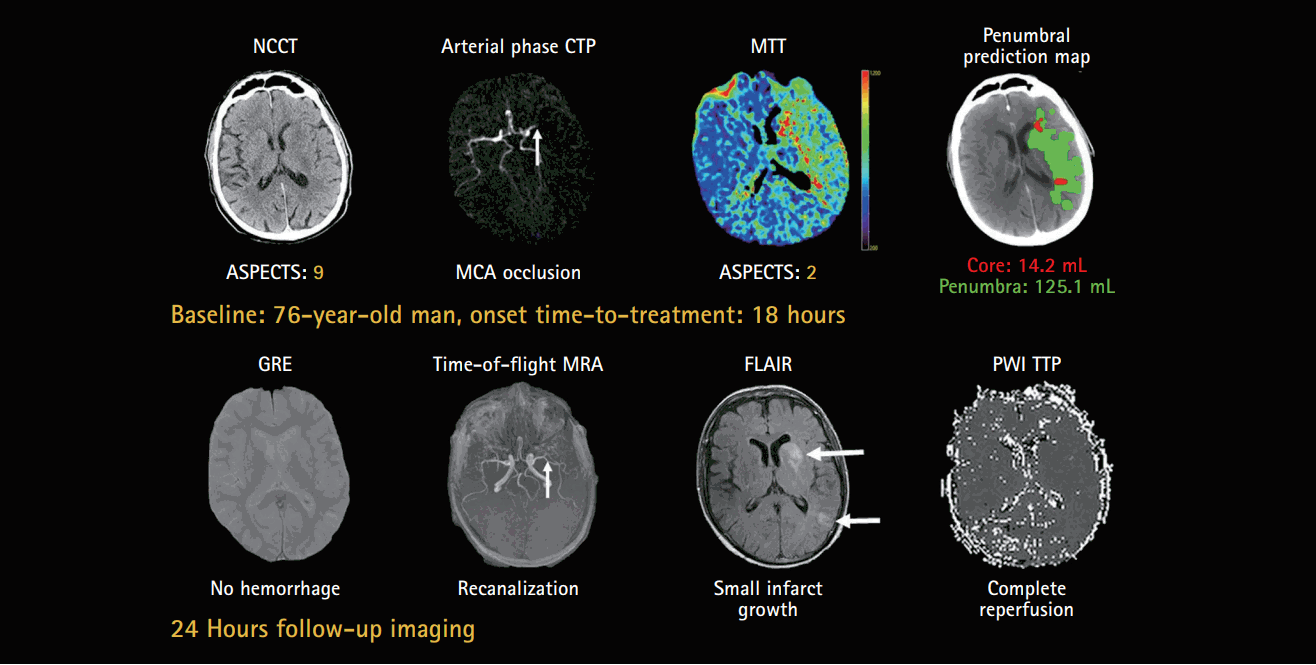
Acute computed tomography perfusion (CTP) maps and 24 hours follow-up perfusion weighted imaging (PWI) in a 74-year-old man presenting 18 hours after onset. The CTP demonstrated a penumbral pattern (infarct core <70 mL, penumbra >15 mL, mismatch >1.8). Tenecteplase was associated with recanalization (vertical arrows) and reperfusion, as well as minimal infarct growth on the magnetic resonance image at 24 hours after treatment (horizontal arrows). NCCT, non-contrast computed tomography; ASPECTS, Alberta Stroke Program Early CT Scores; MCA, middle cerebral artery; MTT, mean transit time; GRE, gradient recalled echo; MRA, magnetic resonance angiography; FLAIR, fluid attenuated inversion recovery; TTP, time to peak.
Patients were excluded if there were contraindications to CTP or PWI, evidence of renal failure (creatinine >160 μmol/L or glomerular filtration rate <50 mL/min), or history of recent stroke (<30 days), intracranial hemorrhage or coagulopathy. Other exclusion criteria were the presence of a known vascular malformation, history of a recent surgical procedure (<21 days), biopsy/subclavian venous/or arterial puncture (<7 days), trauma and gastrointestinal or genitourinary bleeding within 14 days of the event, as well as patients treated with IV heparin within the previous 24 hours. Lab exclusion criteria were international normalized ratio >1.4, platelet count <100,000 and capillary glucose of either <3 or >18 mmol/L.
Treatment groups
After informed consent, patients with mismatch patterns received weight-adjusted IV TNK 0.25 mg/kg, with a maximum dose 25 mg delivered as a bolus [4,8,11]. Two groups of patients were used as comparators. The first group consisted of patients with mismatch patterns who were not treated with reperfusion therapy of any kind, but were enrolled in putative neuroprotective studies. The trial options offered to each patient were made at the discretion of the treating physician and were not randomized. The second group consisted of patients who were not treated with TNK, based on a non-mismatch pattern. All patients received standard guideline-based stroke unit care [12].
Perfusion imaging acquisition
Magnetic resonance imaging
The magnetic resonance imaging (1.5T, Siemens Sonata, Siemens, Munich, Germany) protocol consisted of DWI, gradient recalled echo, fluid attenuated inversion recovery (FLAIR), timeof-flight magnetic resonance angiography (TOF-MRA), and PWI. Perfusion images were derived from the concentration-time curve obtained after the administration of IV gadolinium (0.1 mmol/kg; 5 mL/sec power injection, followed by 15 to 20 mL saline flush at the same rate) with echo planar imaging gradient-echo (T2*) images acquired every 1.4 seconds for 84 seconds (17 axial slices at each of 60 time points).
Computed tomography
A whole brain NCCT (5 mm slice thickness) was followed by CTP with 40 or 80 mm coverage and 5 mm slice thickness acquired every second over 45 seconds (80 kvp; 200 mA/image). Iodinated contrast (40 to 50 mL) was injected at a rate of 5 mL/sec. Extracranial and intracranial computed tomography (CT) angiography was performed after CTP with a separate iodinated contrast injection at 5 mL/sec.
Acute perfusion analysis
Raw PWI (syngo.MR Neuro Perfusion Engine, Siemens) and CTP (NeuroVPCT, Siemens) images were post-processed, using a deconvolution algorithm, immediately after the image acquisition to generate cerebral blood flow (CBF), CBV, and MTT maps [13]. The treating stroke neurologist assessed NCCT or DWI (b=1,000) and post-processed perfusion images, to determine eligibility for TNK treatment. ASPECT scores were assessed on NCCT, CTP maps, DWI and PWI maps as described above [9,10].
Follow-up
Follow-up imaging was obtained 24 hours and 5 days after treatment with TNK. This consisted of an MRI, using all sequences described above. PWI was repeated at 24 hours, but not at 5 days. Those patients with contraindications to MRI underwent a NCCT and CTP at 24 hours and NCCT at 5 days. Patients were clinically reassessed at the time of repeat imaging (24 hours and 5 days) and at day 90 with measurements of neurological deficit (NIHSS score) and functional disability (modified Rankin Scale [mRS] score). Patients who were not treated with TNK underwent follow-up MRI/NCCT without perfusion between 24 to 48 hours as standard-of-care.
Post hoc image analysis
Baseline and follow-up CTP and PWI data performed were post-processed with MIStar software (Apollo Medical Imaging Technology, Melbourne, Australia) to generate CBF, CBV, and delay time (DT) maps. Volumes of ischemic core (region with DT >3 seconds and regional CBF <40% on CTP or diffusion restriction on DWI), penumbra (DT >3 seconds), and total perfusion deficit (core+penumbra) were calculated at baseline [14]. Percent reperfusion was defined as ([total baseline perfusion deficit volume–24 hours perfusion deficit volume] / total baseline perfusion deficit volume) × 100. Penumbral salvage was defined by subtracting final infarct volume from baseline penumbral volume. Final infarct volume (mL) was calculated on 24-hour NCCT/FLAIR using semi-automated volumetric techniques (Quantomo, Cybertrial, and Calgary) and infarct growth was determined by subtracting the ischemic core on acute perfusion imaging from the final infarct volume. Recanalization was classified using the thrombolysis in myocardial infarction (TIMI) scale on the 24 hours follow-up TOF-MRA or CT angiogram [15]. Complete recanalization was defined as TIMI 3 flow and partial recanalization was TIMI ≥1 improvement from baseline.
Outcomes
The primary study outcome was symptomatic HT in patients treated with TNK. Symptomatic HT was defined as the presence of a clinical deterioration (increase in NIHSS ≥4) associated with a parenchymal hematoma (PH) type 1 or 2 (based on ECASS II criteria) within 36 hours from stroke onset [16]. Rating of HT was performed by a neuroradiologist (H.K.), blinded to treatment. Secondary imaging outcomes were any HT on follow-up imaging, percent reperfusion at 24 hours, recanalization in patients treated with TNK, penumbral salvage and infarct growth. Secondary clinical outcome measures were improvement in neurological deficit (NIHSS score) at 24 hours after treatment, and functional outcome at 90 days (mRS score of 0 to 2).
Stopping rule
Symptomatic HT of ischemic infarcts following tPA administration between 0 and 6 hours has been shown to be approximately 8% [5]. In a sample of 20 patients, no more than two cases of symptomatic HT related to thrombolytic therapy would be expected. An a priori stopping rule governed study continuation; planned recruitment would halt if >2 patients develop symptomatic HT.
Statistical analysis
Statistical analysis was performed on SPSS version 22.0 (IBM Corp., Armonk, NY, USA). Differences in median infarct core, penumbra, infarct growth, and penumbral salvage between the three groups (TNK group, untreated mismatch group, and untreated non-mismatch group) were assessed with a one-way analysis of variance (ANOVA), followed by Kruskal-Wallis Post hoc tests for paired comparisons. Differences in the frequency of good functional outcome (mRS score of 0 to 2) between groups were assessed with Pearson chi-square test. Predictors of good functional outcome (mRS 0 to 2) were also assessed with univariate logistic regression. A multivariate logistic regression model was limited to the three strongest predictors of good functional outcome, in order to avoid over-specification.
Results
Patients
A total of 133 patients underwent CTP <24 hours after symptom onset. Seventy-six patients were excluded for reasons outlined in Figure 2. Fifty-seven patients were subsequently included for analysis, and 16 (28.1%) of these patients who met the inclusion and exclusion criteria were treated with TNK. One patient was lost to follow-up.

Study screening and enrollment profile. IA, intra-arterial; NIHSS, National Institutes of Health Stroke Scale.
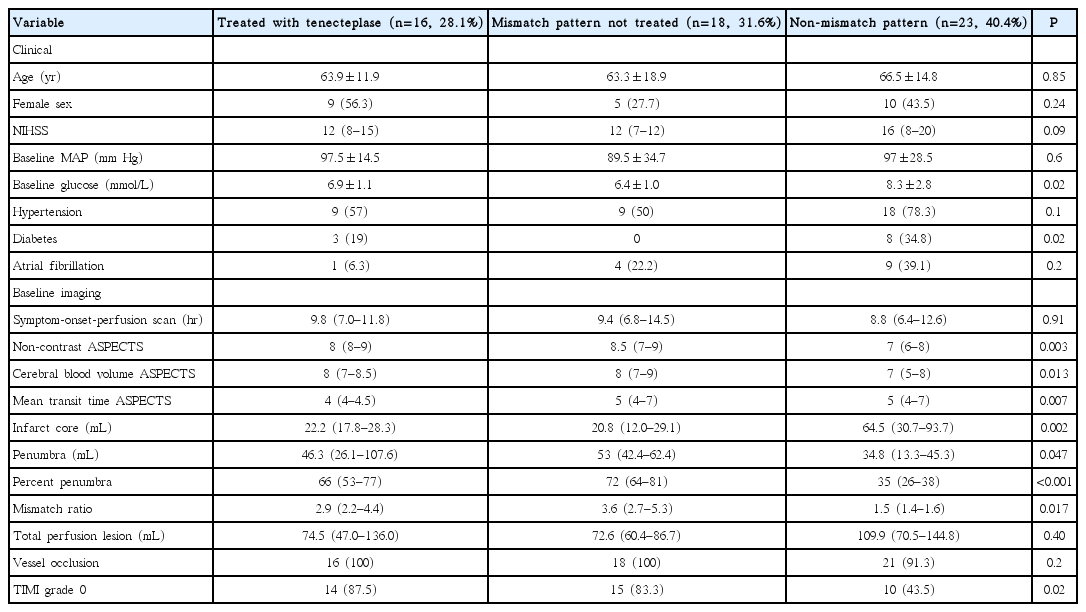
Forty-one patients not treated with TNK were in the comparator groups. Eighteen patients (31.6%) had a mismatch pattern but were enrolled in other trials (non-endovascular and non-thrombolysis), and 23 patients (40.4%) did not meet the criteria for a penumbral patterns, and therefore were not treated. More patients in the non-mismatch pattern untreated group were diabetic, with a higher median blood glucose level. Otherwise, baseline clinical characteristics were similar among the three groups (Table 1).
Baseline imaging
Four patients (25.0%) in the TNK group underwent baseline PWI and the remainder were assessed with CTP. The median time from symptom-onset to perfusion imaging was 9.8 hours (interquartile range [IQR], 7.0 to 11.8). The total hypoperfused region (assessed with MTT ASPECT scores) was comparable among groups. ASPECT scores of infarct core on the baseline NCCT and CBV maps were significantly lower in patients with non-mismatch patterns (Table 1).
When measured planimetrically, there was no difference in the total perfusion deficit volumes, assessed with MTT, among groups (Table 1). The median (IQR) baseline infarct core varied between the mismatch patients treated with TNK (22.2 mL [IQR, 17.8 to 28.3]) and those with a mismatch pattern but enrolled in other trials (20.8 mL [IQR, 12.0 to 29.1]) and those with non-mismatch patterns (64.5 mL [IQR, 30.7 to 93.7]; ANOVA P=0.002). Non-mismatch untreated patients had a larger baseline core than both the untreated mismatch (P=0.004) and the TNK treated patients (P=0.023).
Treatment
Patients were treated with TNK at a median 9.6 hours (range, 8.2 to 11.5) after symptom onset with a range of 5.1 to 23.7 hours. The mean dose of TNK given was 18.5±4.6 mg (range, 7.4 to 25). In addition to TNK, two patients received endovascular mechanical aspiration therapy. All other patients were treated with acetylsalicylic acid within 24 hours of onset, but no other antithrombotic agents or reperfusion therapies.
Primary outcome: hemorrhagic transformation
Symptomatic intracranial hemorrhage occurred in one patient (6.3%) treated with TNK, and this patient had parenchymal hemorrhage type 2. Three patients (18.8%) developed asymptomatic parenchymal hemorrhage type 1 and two patients (12.5%) developed hemorrhagic infarction (HI).
In the untreated mismatch group, one patient (5.6%) developed HI type 1 and one (5.6%) other patient developed PH type 2. In the untreated non-mismatch group, four patients (17.4%) developed HI type 1, three patients (13%) developed HI type 2, and one patient (4.3%) developed PH type 2.
Secondary imaging outcomes
Resolution of >80% and >50% of the baseline perfusion deficit was seen in 9 (56.3%) and 10 (62.5%) TNK treated patients respectively. Complete and partial recanalization was observed in 8 (50.0%) and 2 (12.5%) TNK treated patients.
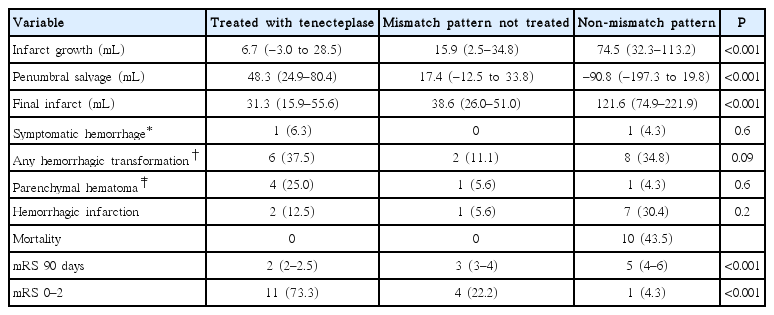
Median infarct growth varied between the TNK treated patients (6.7 mL [range, –3.0 to 28.5]), the untreated mismatch patients (12.9 mL [range, 2.5 to 34.8]), and the non-mismatch patients (74.5 mL [range, 32.3 to 113.2]; ANOVA P<0.001). TNK treated patients had smaller median infarct growth than untreated non-mismatch patients (P<0.001). The penumbral salvage volume also differed in TNK treated patients (48.3 mL [range, 24.9 to 80.4]) compared to untreated mismatch (17.4 mL [range, –12.5 to 33.8]) and non-mismatch (–90.8 mL [range, –197.3 to 19.8]; ANOVA P<0.001) patients. Untreated non-mismatch patients had smaller median penumbral salvage than TNK treated patients (P<0.001).
Clinical outcomes
Median NIHSS score at 24 hours in the TNK treated group was 7 (range, 5 to 11), and 9 patients (56.3%) had an improvement in NIHSS score by ≥4 points. The median mRS score at 90 days was lower in the TNK group (2 [range, 2 to 2.5]) than the untreated mismatch (3 [range, 3 to 4]; odds ratio [OR], 2.4; 95% confidence interval [CI], 1.1 to 5.3; P=0.03) and non-mismatch (5 [range, 4–6]; OR, 4.5; 95% CI, 1.6 to 12.8; P=0.005) patients (Table 2). The proportion of patients with mRS 0 to 2 was significantly higher in the TNK group (73.3%) than untreated mismatch (22.2%) and non-mismatch (4.3%, P<0.001) groups. There was no deaths in the TNK treated and untreated mismatch groups. Ten patients in the non-mismatch group died.
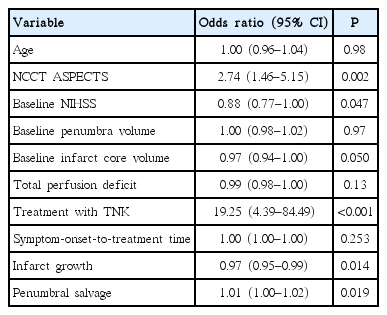
Univariate logistic regression indicated that good functional outcome was associated with TNK treatment, baseline NCCT ASPECTS, NIHSS scores, infarct growth and penumbral salvage (Table 3). A multivariable logistic regression model including TNK treatment, baseline NCCT ASPECTS and NIHSS scores was built. This model indicated treatment with TNK (OR, 35.7; 95% CI, 4.0 to 317.0; P=0.001) and NCCT ASPECTS (OR, 3.4; 95% CI, 1.2 to 9.8; P=0.026) remained as independent predictors of good functional outcome.
Discussion
This pilot study supports the safety and feasibility of TNK for ischemic stroke patients outside of the approved thrombolysis treatment windows, in patients with evidence of a penumbral pattern. Treatment with TNK was associated with early reperfusion, recanalization and penumbral tissue salvage. These surrogate outcome measures were associated with early clinical recovery at 24 hours and good functional outcome at 90 days.
Tenecteplase in ischemic stroke
There is some evidence to suggest TNK is superior to tPA in the treatment of acute ischemic stroke. A recent randomized trial demonstrated that TNK (0.1 and 0.25 mg/kg) was associated with a higher reperfusion rate and improved clinical outcomes over tPA [4]. As in our study, patients were selected on the basis of a penumbral pattern on perfusion imaging, although treatment was limited to those presenting within six hours of symptom onset. In another study, TNK was found to result in recanalization in patients with persisting middle cerebral artery occlusions despite tPA treatment [17].
In our study, one patient (6.3%) developed symptomatic HT. This HT rate seems comparable to previous trials of TNK in ischemic stroke. The first published study of TNK in ischemic stroke indicated that symptomatic HT occurred in 0%, 6.5%, and 15.8% at doses of 0.1, 0.25, and 0.4 mg/kg, respectively [11]0 In a randomized trial in patients with a penumbral pattern within 6 hours of onset, symptomatic HT occurred in 2/25 TNK treated patients (8%) and 3/25 tPA treated patients (12%) [18]. Finally, a recent open label pilot study indicated that TNK in minor ischemic stroke (NIHSS score <6) was associated with a symptomatic HT rate of 4% (1/25 patients treated with 0.25 mg/kg) [19].
Thrombolysis in patients with unknown onset times
Several studies have assessed the feasibility of thrombolysis with tPA in patients with unknown time of onset. Selection criteria between these studies varied widely, as did treatment response rates [20-26]. Most of these studies did not utilize penumbral selection paradigms. Good functional outcome rates (mRS 0 to 2) in patients treated with tPA with unknown onset (<24 hours of last known well) was between 35% and 50% [21-24]. Currently, ischemic stroke patients presenting more than 4.5 hours from onset/last known well and without a large-vessel occlusion have no acute reperfusion treatment options. In contrast, the therapeutic window for patients with proximal occlusions is anywhere from 6 to 12 hours, based on published trial data [27]. Our results support IV thrombolysis treatment of patients with more distal occlusions, who present >4.5 hours after onset. Although we assessed for an ischemic penumbra with CTP, it is possible that TNK may be useful in patients selected without advanced imaging, as the NCCT ASPECTS also predicted outcome (Table 3).
Rapid penumbral pattern assessment
At the time this protocol was developed, automated perfusion threshold and planimetric software analysis programs were unavailable [4,28]. In order to expedite interpretation of perfusion maps, we utilized ASPECTS applied to perfusion maps. ASPECTS have been successfully used to interpret post-processed CTP maps in retrospective studies [9,29]. This is the first study in which ASPECTS was used prospectively. Our Post hoc planimetric analysis of bolus delay and dispersion corrected perfusion maps confirmed that all patients with ASPECTS mismatch >2 had penumbral patterns. Nonetheless, we acknowledge that automated post-processing and planimetric measurement of perfusion volumes is the preferred method for selecting patients on the basis of blood flow maps.
Limitations
This study is limited by the small sample size and non-randomized design. The original aim of the study was to demonstrate safety of TNK in 20 patients, as a pilot phase for a randomized trial of TNK versus placebo in patients with penumbral patterns presenting >4.5 hours after onset. The study was terminated due to slow recruitment secondary to competing non-interventional trials (our first comparator group of patients) followed by an endovascular treatment trial, which permitted enrollment of patients with large artery occlusions up to 12 hours after onset [30]. Endovascular therapy is now standard therapy for patients with large vessel occlusions at our hospital, and indeed this intervention was a confounder in two of the TNK-treated patients in our study. Ultimately, however, TNK followed by endovascular therapy may be the ideal approach to patients with proximal occlusions.
Conclusions
This small study demonstrates only the feasibility of treating patients far outside of established treatment windows with a novel thrombolytic agent. There is now strong evidence that patients with large vessel occlusions respond to endovascular clot retrieval up to 12 hours after onset [27]. The role of thrombolytics >4.5 hours as a bridge to endovascular treatment and/or in patients without occlusions amenable to clot retrieval remains unknown. The high rate of recanalization and low rate of hemorrhagic complications seen in this and other studies suggest that TNK may be valuable in these settings.
Notes
Disclosure
The authors have no financial conflicts of interest.
Acknowledgements
This trial was funded using research grants provided to the Principal Investigator through the Canada Research Chair program, as well as the Heart and Stroke Foundation of Alberta, Northwest Territories and Nunavut Professorship in Stroke Medicine. Neither of these organizations had any role in study design, data collection, analysis, interpretation, or manuscript preparation. TNK was provided by Roche Canada. Mahesh Kate and Laura C. Gioia have AIHS Clinician Fellowship Award. Parnian Riaz received AIHS Summer Student Scholarship.