Correlation of Adventitial Vasa Vasorum with Intracranial Atherosclerosis: A Postmortem Study
Article information
Abstract
Background and Purpose
Vasa vasorum (VV) have been believed to be rare or non-existent in small-caliber intracranial arteries. In a series of human cerebral artery specimens, we identified and examined the distribution of VV in association with co-existing intracranial atherosclerosis.
Methods
We obtained cerebral artery specimens from 32 consecutive autopsies of subjects aged 45 years or above. We scrutinized middle cerebral artery (MCA), vertebral artery (VA), and basilar artery (BA) for the presence of adventitial VV. We described the distribution of VV, and the characteristics of co-existing atherosclerotic lesions.
Results
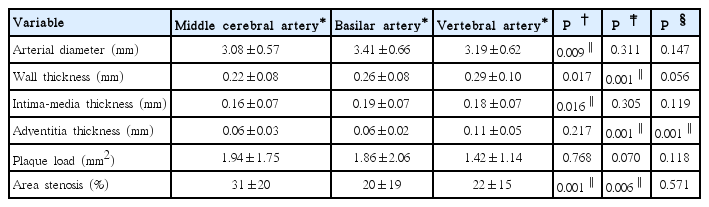
Among 157 intracranial arteries, adventitial VV were present in 74 of the 157 specimens (47%), involving MCA (n=13, 18%), BA (n=14, 19%), and VA (n=47, 64%). Although qualitatively these 74 adventitial VV distributed similarly in arteries with or without atherosclerotic lesions (disease-free arteries n=4/8; arteries of pre-atherosclerosis n=17/42; and arteries of progressive atherosclerosis n=53/107), the presence of adventitial VV in intracranial VA was associated with a heavier plaque load (1.72±1.66 mm2 vs. 0.40±0.32 mm2, P<0.001), severer luminal stenosis (25%±21% vs. 12%±9%, P=0.002), higher rate of concentric lesions (79% vs. 36%, P=0.002), and denser intraplaque calcification (44% vs. 0%, P=0.003). Histologically, intracranial VA with VV had a larger diameter (3.40±0.79 mm vs. 2.34±0.58 mm, P<0.001), thicker arterial wall (0.31±0.13 mm vs. 0.23±0.06 mm, P=0.002), and a larger intima-media (0.19±0.09 mm vs. 0.13± 0.04 mm, P=0.003) than VA without VV.
Conclusions
Our study demonstrated the distribution of adventitial VV within brain vasculature and association between vertebral VV and progressive atherosclerotic lesions with a heavier plaque load and denser intraplaque calcification.
Introduction
Atherosclerosis is a chronic progressive disease characterized by lipid accumulation in the vascular wall and fibrous cap formation [1,2]. Intracranial atherosclerosis is a major cause of stroke and may account for 30% to 50% of ischemic events in Asians [3-5]. As a critical contributor to the development of atherosclerosis, inflammation has been shown to progress inward from adventitia to intima [2,6,7].
Histologically, adventitia consists of connective tissue, fibroblasts, and vasa vasorum (VV) [1]. VV constitute a network of microvasculature and play a nutritive role with drainage capacity in the arterial vessel walls [8]. Studies have shown that VV may function as a conduit for transporting inflammatory mediators [9-11], and therefore, may play a passive role in the pathogenesis of atherosclerosis, aneurysm, vasculitis, and graft vascular disease [11,12]. Adventitial VV in coronary and carotid arteries may contribute to the vulnerability of atherosclerotic plaques [10]. However, the ability of cerebral arteries to receive nourishment from surrounding cerebrospinal fluid may help explain the relative absence of intracranial VV from early life [13].
Previous autopsy studies reported intracranial VV by immunohistochemical examination [14,15]. Nevertheless, compared with the extracranial VV, knowledge on the histopathology of intracranial VV is scarce, partly owing to the relative inaccessibility of intracranial arteries. Understanding adventitial VV development in cerebral arteries and its relationship to atherosclerosis may shed light on mechanisms of ischemic stroke due to presumed intracranial atherosclerosis. Based on a series of human cerebral artery specimens in our biobank [16-19], we investigated the distribution of adventitial VV in intracranial large arteries and studied its potential association with co-existing atherosclerosis.
Methods
Participants
We selected 32 Chinese autopsies from December 2003 to June 2005 in the Prince of Wales Hospital, Hong Kong [16]. Clinical Research Ethics Committee of the Chinese University of Hong Kong approved the study. Pathologists who performed the autopsy were blinded to the study purpose. We obtained subject demographics and clinical data from hospital electronic records. The causes of death were cardiovascular disease (n=13, 41%, e.g., coronary artery disease, hypertensive heart disease, ischemic stroke, or brain hemorrhage); infection or sepsis (n=3, 9%); other natural causes (n=13, 41%, e.g., hepatitis); or unnatural causes (n=3, 9%, e.g., suicides or accidents).
Histopathology
We sampled 157 intracranial arteries from 32 autopsy cases, including M1 segments of bilateral middle cerebral arteries (MCA, n=64), basilar artery (BA, n=32), and V4 segments of bilateral vertebral arteries (VA, n=61; three individuals had a single VA). We obtained and labeled each 4 mm cross-sectional cut of cerebral arteries from formalin-fixed brains. Each segment was decalcified overnight in 10% formic acid, followed by perfusion fixation in fresh 30% formaldehyde. After embedding in paraffin, five-micron-thick cuts were obtained from each arterial block for staining (one section per staining) with hematoxylin and eosin (H&E) for structural evaluation. We used Victoria Blue staining to mark the internal elastic lamina (IEL) for morphological measurements. For immunohistochemistry, we used Abcam (Cambridge, UK) antigen retrieval solution to retrieve antigen. The sections were then incubated with 3% hydrogen peroxide for 15 minutes and bovine serum albumin for 1 hour and stained with the following primary antibodies: von Willebrand factor (vWF) (1:200, Agilent Dako, Santa Clara, CA, USA) and CD68 (1:200, Sigma, St. Louis, MO, USA).
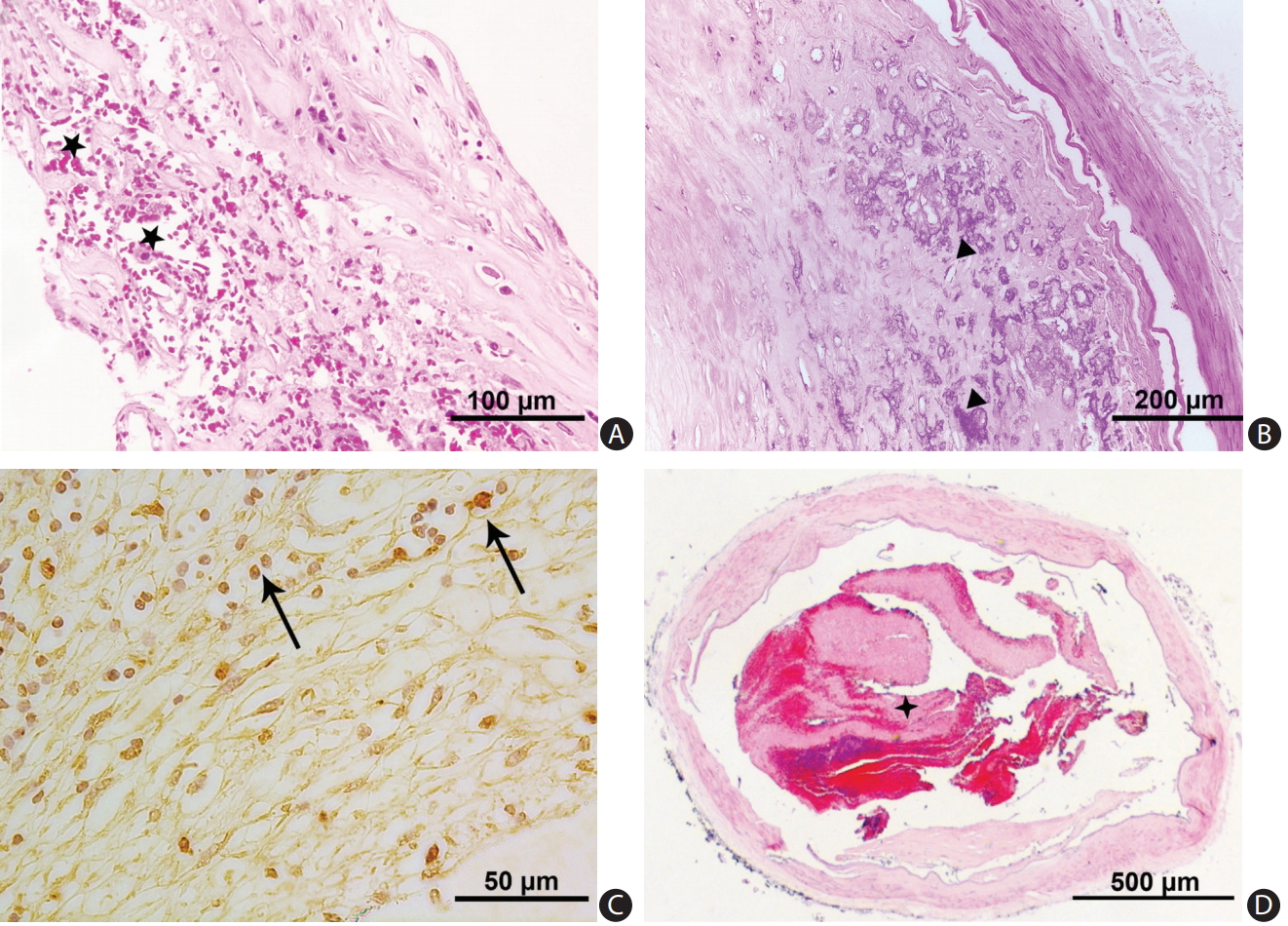
We photographed the slides with a Leica DC 200 digital microscope (Leica, Witzlar, Germany). Two pathologists (H.L.Z. and C.B.N.) blinded to clinical data examined all histological sections to grade atherosclerotic lesions and to record the plaque features. Another two investigators (L.Z. and W.J.Y.) independently located and described the distribution of VV: adventitial VV were identified by H&E staining (Figure 1A-C and Supplementary Figure 1), as well as immunostaining that outlined the diffuse perivascular deposits of vWF surrounding a microvessel (Figure 1D). Based on the revised American Heart Association (AHA) criteria [20], we classified the arteries into three groups: (1) disease-free, with normal intima; (2) pre-atherosclerotic lesions showing intimal thickening or intimal xanthoma; and (3) progressive atherosclerotic lesions showing pathologic intimal thickening, fibrous cap atheroma or fibrocalcific plaque. Concentric lesions were plaques involving the entire circumference of the ILE, whereas eccentric plaques had intervening diseasefree wall [21]. We recorded the characteristics of a complicated plaque, if any, including intraplaque hemorrhage (Figure 2A), calcification (Figure 2B), macrophage infiltration (Figure 2C), and thrombus (Figure 2D).
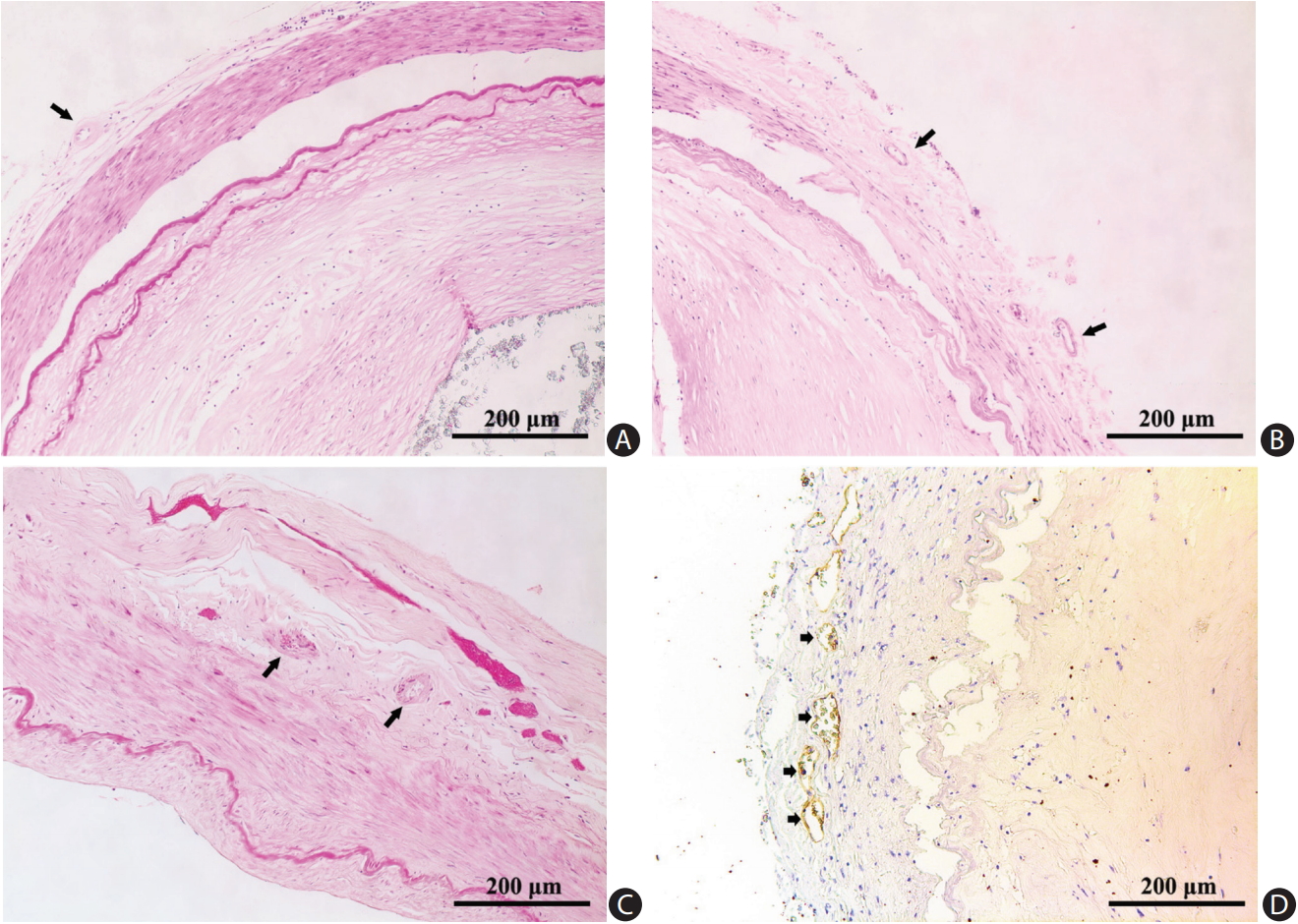
Vasa vasorum in adventitia of cerebral arteries (arrows). (A) Middle cerebral artery (H&E stain). (B) Basilar artery (H&E stain). (C) Vertebral artery (H&E stain). (D) Immunochemical staining for von Willebrand factor revealed multiple vasa vasorum (short arrows).
Image analysis
We used Image-Pro Plus software to assess arterial structure. Based on the method described by Gutierrez et al. [22], we measured arterial diameter, wall thickness, intima-media thickness, adventitia thickness, area of atherosclerotic plaque (plaque load), and percentage of luminal narrowing (area stenosis). We traced the border of external adventitia, media, IEL and plaque manually and calculated the length and area with software. After outlining the perimeters of different layers as Pouter, Pmedia, and PIEL, we calculated Rartery, Rmedia, and RIEL by radius = perimeter / 2π. The thicknesses (Th) of adventitia and intima-media were calculated as follows: Thadventitia = Rartery – Rmedia, Thintima-media = Rmedia – RIEL. The wall thickness was the sum of Thadventitia and Thintima-media. We derived the luminal area by AIEL= PIEL2 / 4π, and the area stenosis by (plaque load / AIEL) × 100.
Statistical analysis
We analyzed data using SPSS version 20.0 software package (IBM Co., Armonk, NY, USA). For comparisons among different cerebral arteries for the 32 subjects, we used mean values of the MCAs and VAs for a given autopsy case and comparisons were made using chi-square test for categorical data and paired t-test for continuous variables. Comparisons between arteries with and without adventitial VV were made by independent sample t-test for continuous variables and chisquare test for categorical variables. Results are presented as mean±SD. We considered P<0.05 as statistically significant. For multiple testing, a significance level was considered as 0.016 (P=0.05/3) by Bonferroni correction.
Results
Sample description
We selected all 32 Chinese adult autopsies aged 45 years or above from December 2003 to June 2005. Median age was 71 years (range, 45 to 97). Twenty-three (72%) were male. For cardiovascular risk factors, smoking was found in nine cases (28%), hypertension in nine (28%), and diabetes mellitus in six (19%). For the history of clinical cardiovascular events, nine had ischemic heart disease (28%), 14 had ischemic stroke (44%), and two had hemorrhagic stroke (6%). Table 1 summarizes the demographics and risk factors of the included cases.
Anatomy and distribution of adventitial VV
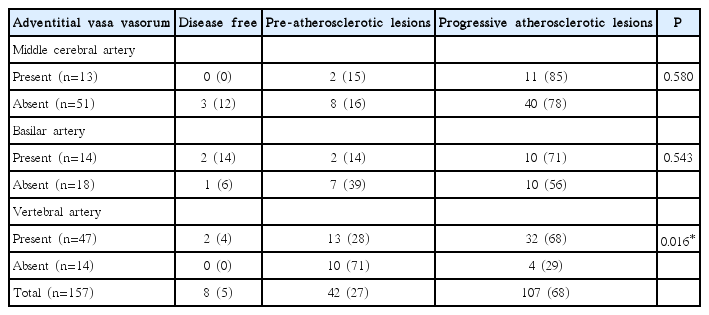
We scrutinized 157 intracranial arteries: 64 MCAs, 32 BAs, and 61 VAs. Table 2 shows comparison of histologic features among different intracranial arteries. MCA had a thinner arterial wall than VA (0.22±0.08 mm vs. 0.29±0.10 mm, P<0.016). VA had a significantly thicker adventitia (0.11±0.05 mm, P<0.016) than MCA or BA. BA had a larger artery diameter (3.41±0.66 mm vs. 3.08±0.57 mm, P<0.016) and a thicker intima-media (0.19±0.07 mm vs. 0.16±0.07 mm, P<0.016) than MCA. The area stenosis (%) was greatest in MCA compared with BA or VA (31%±20% vs. 20%±19% or 31%±20% vs. 22%±15%; P<0.01, respectively). Adventitial VV were most prevalent in VA (47/61) compared with MCA (13/64) or BA (14/32) (77% vs. 20% or 77% vs. 44%; P<0.016, respectively).
Association between VV and phenotypes of atherosclerosis
Based on the revised AHA criteria, eight arteries (5%) were disease-free with normal intima, 42 (27%) had pre-atherosclerotic lesions, and 107 (68%) had progressive atherosclerotic lesions. Among all 157 arterial distributed segments, we identified adventitial VV in 74 specimens (47%): 13 MCAs (18%), 14 BAs (19%), and 47 VAs (64%). In terms of co-existing atherosclerosis, these 74 adventitial VV were present in four disease-free segments (5%), in 17 segments with pre-atherosclerotic lesions (23%), and in 53 segments with progressive atherosclerotic lesions (72%). In MCA and BA, the prevalence of atherosclerotic lesions did not significantly differ between arteries with adventitial VV and those without. However, in VA, arteries with adventitial VV were likely to have more co-existing progressive atherosclerotic lesions compared to arteries without adventitial VV (68% vs. 29%, P<0.05) (Table 3).
Association between adventitial VV and atherosclerotic surrogates in VA
Table 4 compares the anatomy and atherosclerotic features in VA with and without VV. The VA with adventitial VV had a larger diameter (3.40±0.79 mm vs. 2.34±0.58 mm, P<0.001), a thicker arterial wall (0.31±0.13 mm vs. 0.23±0.06 mm, P=0.002), and a thicker intima-media (0.19±0.09 mm vs. 0.13±0.04 mm, P=0.003) than VA without VV. The adventitial VV in V4 segments was associated with a heavier plaque load (1.72±1.66 mm2 vs. 0.40±0.32 mm2, P<0.001), severer area stenosis (25%±21% vs. 12%±9%, P=0.002), higher rate of concentric lesions (79% vs. 36%, P=0.002), and denser intraplaque calcification (43% vs. 0%, P=0.003).
Discussion
In this autopsy study, we found adventitial VV in nearly half (74/157, 47%) of all intracranial arterial segments, predominantly in V4 segments of VA (64%), followed by BA (19%) and MCA (18%). Overall, adventitial VV were present in disease-free arteries (4/8, 50%) as well as in arteries with pre-atherosclerotic (17/42, 40%) or progressive atherosclerotic lesions (53/107, 50%). In VA, adventitial VV were associated with concentric steno-occlusive atherosclerotic lesions and denser intraplaque calcification.
VV are composed of artery, capillary, and vein that deliver oxygen and nutrition to and eliminate metabolic wastes from the vessel wall [23,24]. In contrast to the high prevalence of VV in extracranial arteries, the existence of VV in human intracranial arteries had been a debate due to paucity of cerebral specimens. In 1980s, although Zervas et al. [25] and Clower et al. [26] reported no VV in cerebral arteries in animal studies, VV were revealed in adventitia of internal carotid artery (ICA), anterior cerebral artery and MCA of five humans, suggesting that VV might be species-specific [14]. In a larger autopsy series of 15 cases aged 5 days to 86 years, Aydin [15] subsequently revealed the presence of VV in proximal intracranial segments of ICA and VA but not in BA or MCA. However, in the current study of a larger sample size, we noted that VV were frequently found in the vertebral-basilar circulation (80% in V4 segments of VA and 44% of BA), but much less in MCA (only 20%).
In our study, the adventitia of VA was significantly thicker than that of MCA and BA, and thus, the higher prevalence of VV in VA might correspond to a higher metabolic demand in a thicker vessel wall where diffusion from parent arterial lumen or CSF for nutrition or oxygen might be insufficient. A previous study reported that the extent and distribution of VV depended on the medial thickness [27]. In fact, arterial wall and intima-media layers in VA with adventitial VV were found to be thicker than those without. Our findings along with literature [14,15] suggest that adventitial VV might exist more frequently in the proximal parts of the intracranial arteries such as VA than in arteries located more distally. As a natural extension of extracranial arteries, VA is likely a transition zone that possesses certain features different from the true intracranial arteries such as MCA and BA.
Previous studies suggested that VV were extremely rare in cerebral arteries and might develop only in pathological conditions [28]. Our study findings do not substantiate this theory. On the contrary, adventitial VV were present in four disease-free arteries from two autopsies devoid of any vasculopathy. Nevertheless, while this finding might support the physiologic existence of VV in cerebral arteries, it remains unclear whether VV could trigger and exacerbate the development and progression of atherosclerosis.
In 1984, Barger et al. [29] hypothesized that VV could be involved in the process of atherosclerosis. Studies in both human autopsy [30] and animal models [31] found higher density of VV in unstable atherosclerotic lesions. In a coronary artery disease model, VV may trigger the initial stage of atherosclerosis [32]. In our study, a higher prevalence of progressive atherosclerotic lesions in V4 segments, with heavier plaque load and severer luminal stenosis were found in conjunction with adventitial VV. We postulate that adventitial neovascular network might act as conduits transporting inflammatory cells and mediators into the plaque, exacerbating the pathologic process of atherosclerosis [7,23]. In this current study, we found that cerebral arteries with VV were more likely to have atherosclerotic plaques with more hemorrhage. Immature microvessels could act as sites of inflammatory cell infiltration and intraplaque hemorrhage owing to the weak integrity of such vessels [33,34]. Studies in coronary artery and carotid showed that intraplaque hemorrhage is not only a common feature in advanced atherosclerotic plaques [35], but also plays an important role in plaque destabilization which ultimately may lead to clinical ischemic events [12,36-38]. In cerebral arteries, our previous findings demonstrated that in addition to the luminal stenosis, plaque components such as lipid and intraplaque hemorrhage may be responsible for the brain infarct [16]. Therefore, adventitial VV may play a nutritive role in the progression of intracranial atherosclerosis and might be considered as a marker of complicated plaques. However, whether adventitial VV in intracranial arteries play a causative role in brain infarction has not been well established yet.
The interpretation of our findings would be limited by the selection bias inherent to a retrospective post-mortem study. The heterogeneity of the enrolled autopsy cases could be a major source of confounder and extrapolation to the general population should be treated with caution. Considering the nature of a post-mortem study, we could not provide causal relationship between presence of VV and progression of atherosclerosis within the intracranial vascular beds. Besides, we did not analyze the association between the magnitude of adventitia VV and atherosclerosis in this study. Moreover, although our study has a relatively larger sample size compared with previous studies, the number was still insufficient to allow subgroup analysis to analyze adventitial VV in relation to various stages of atherosclerosis and to adequately adjust covariates. Future investigation may shed light on whether and how adventitial VV may impact on the evolution of atherosclerosis.
Conclusions
In conclusion, our investigation shows high prevalence of adventitial VV in intracranial arterial segments of VAs. In VAs, we observed an association between VV and progressive atherosclerotic lesions with a heavier plaque load and denser intraplaque calcification.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2018.01263.
(A, B) Vasa vasorum (triangles) of intracranial vertebral artery. These were cross-sections of two vertebral arteries showing vasa vasorum in close anatomic relation with atherosclerotic plaques (H&E stain, ×1.6).
Notes
Disclosure
The authors have no financial conflicts of interest.
Acknowledgements
The study was supported by grants from the National Natural Science Foundation of China (Project No. 81371297), the General Research Fund from Research Grants Council of Hong Kong (GRF, Project No. 14112916) and the Health and Medical Research Fund of Hong Kong (HMRF, Project No. 04152586). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.