Elevation of the Gut Microbiota Metabolite Trimethylamine N-Oxide Predicts Stroke Outcome
Article information
Dear Sir:
Trimethylamine N-oxide (TMAO) is produced from dietary intake of choline, phosphatidylcholine, and L-carnitine. Recent clinical studies suggest a correlation between elevated plasma TMAO level and increased risk of cardiovascular disease and major adverse cardiovascular events (MACE) [1]. However, reports of patients with stroke are scant and contradictory. Here, we explored the relationship between TMAO level and stroke outcome.
We retrospectively analyzed data from a prospectively enrolled stroke registry. We included first-ever stroke patients with large artery atherosclerosis, cardioembolism, or lacunar infarction. Those with stoke of undetermined, or other determined cause were excluded. The Institutional Review Board approved the study and informed consent was obtained from all patients. Overnight fasting blood samples were collected on 1 day after admission. Blood was drawn by venipuncture and collected in EDTA at 4°C. Within 60 minutes of collection, blood samples were centrifuged at 4,000 rpm for 10 minutes to isolate plasma, then stored at –80°C until testing. Analysis of TMAO was carried out using liquid chromatography-tandem mass spectrometry (LC-MS/MS). Intra-assay imprecision of TMAO quantification was evaluated from five replicates measured in a single series and inter-assay imprecision was obtained from four different assays over 4 days using four different concentrations of quality control materials spiked with TMAO. Intra-assay coefficients of variation (CVs) (n=5) were 1.2% to 4.2% and inter-assay CVs (n=4) were 2.1% to 6.0%. It was also confirmed that repeated freezing and thawing (three cycles) of plasma samples, spiked with TMAO at two different levels, did not affect their stability; mean percent recovery against spiked concentrations was 98.6 and 93.5%.
After discharge, all patients were regularly followed up at 3 months and 1 year, and at each subsequent year. At each follow-up visit, information was obtained regarding any MACE occurrence (recurrent stroke, myocardial infarction, or death). If a patient missed a visit, information was obtained from the patient or a close relative by telephone interview, using a structured questionnaire. For patients who were lost to follow-up, mortality data was obtained from the Korean National Statistical Office.
Statistical analyses were performed using the R package, version 3.1.3 (https://cran.r-project.org). Survival analyses were conducted using Kaplan-Meier and Cox proportional hazard analyses. Observation periods were truncated at 3 years. The best cut-off for TMAO level was calculated using Contal and O’Quigley’s method, which maximizes the log-rank test statistics. Multivariate analysis was performed with adjustment for parameters that were significant in univariate analyses (P<0.05) and blood parameters that were associated with TMAO concentration.
Of 567 patients with available blood samples, 242 stroke patients of undetermined cause, and 12 patients of other determined cause were excluded. Finally, 313 patients were included in the study (median age 68 years: interquartile range [IQR], 57 to 75 years; 62% men). Median TMAO level was 4.8 μmol/L (IQR, 2.8 to 10.0). Patients were categorized into two groups according to the best TMAO cut-off value for predicting MACE (low TMAO group: TMAO <7.5 μmol/L; high TMAO group: TMAO ≥7.5 μmol/L). There were more men in the high TMAO group than in the low TMAO group (75% vs. 55.9%, P=0.002); other demographic characteristics were similar.
During follow-up, (mean 1.9±1.1 years), 72 (23%) MACE were reported and occurrences were significantly higher in the high TMAO group (31%) than in the low TMAO group (19.2%, P=0.031). Kaplan-Meier survival analysis revealed that MACE was more likely in the high TMAO group than in the low TMAO group (P=0.02) (Figure 1). Multivariate analysis revealed that TMAO was an independent predictor of MACE (hazard ratio, 1.690; 95% confidence interval, 1.030 to 2.771), along with age, diabetes, initial National Institutes of Health Stroke Scale score, and hemoglobin level (Table 1).
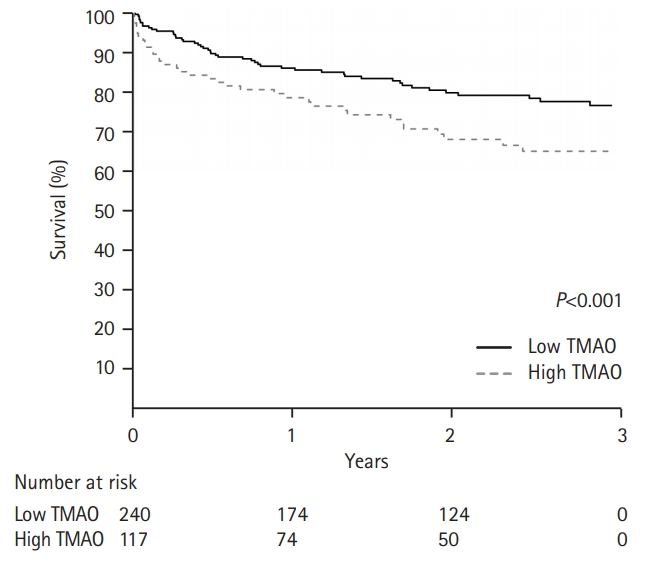
Kaplan-Meier survival analysis for the occurrence of a major adverse cardiovascular event according to trimethylamine N-oxide (TMAO) level.
We demonstrated that TMAO level was an independent predictor of MACE in patients with first-ever stroke. This relationship was observed even after adjustment for traditional risk factors and initial stroke severity.
A previous study showed elevated TMAO levels are dose-dependently associated with an increased risk of recurrent stroke and subsequent cardiovascular events in patients with first-ever stroke [2]. However, a case-control study of Chinese patients with stroke and transient ischemic attack (TIA) reported low TMAO plasma concentrations. The authors explained that they examined TMAO levels in patients who had already suffered from stroke or TIA. Blood TMAO levels were low compared with a study in a Western population, and stroke or TIA treatment may have reduced levels of TMAO [3].
Inconsistencies in results between studies may be related to differences in study methods (e.g., prospective follow-up vs. case-control study). As the present study had a relatively small sample size and possible selection bias, our results should be confirmed in larger studies that include different ethnic groups. In conclusion, TMAO is a potential biomarker for the prediction of MACE after stroke.
Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) grant, funded by the Korean government (MSIT) (2019R1H1A1079907).
Notes
The authors have no financial conflicts of interest.
