General Anesthesia versus Conscious Sedation in Mechanical Thrombectomy
Article information
Abstract
Background and Purpose
Anesthesia regimen in patients undergoing mechanical thrombectomy (MT) is still an unresolved issue.
Methods
We compared the effect of anesthesia regimen using data from the German Stroke Registry-Endovascular Treatment (GSR-ET) between June 2015 and December 2019. Degree of disability was rated by the modified Rankin Scale (mRS), and good outcome was defined as mRS 0–2. Successful reperfusion was assumed when the modified thrombolysis in cerebral infarction scale was 2b–3.
Results
Out of 6,635 patients, 67.1% (n=4,453) patients underwent general anesthesia (GA), 24.9% (n=1,650) conscious sedation (CS), and 3.3% (n=219) conversion from CS to GA. Rate of successful reperfusion was similar across all three groups (83.0% vs. 84.2% vs. 82.6%, P=0.149). Compared to the CA-group, the GA-group had a delay from admission to groin (71.0 minutes vs. 61.0 minutes, P<0.001), but a comparable interval from groin to flow restoration (41.0 minutes vs. 39.0 minutes). The CS-group had the lowest rate of periprocedural complications (15.0% vs. 21.0% vs. 28.3%, P<0.001). The CS-group was more likely to have a good outcome at follow-up (42.1% vs. 34.2% vs. 33.5%, P<0.001) and a lower mortality rate (23.4% vs. 34.2% vs. 26.0%, P<0.001). In multivariable analysis, GA was associated with reduced achievement of good functional outcome (odds ratio [OR], 0.82; 95% confidence interval [CI], 0.71 to 0.94; P=0.004) and increased mortality (OR, 1.42; 95% CI, 1.23 to 1.64; P<0.001). Subgroup analysis for anterior circulation strokes (n=5,808) showed comparable results.
Conclusions
We provide further evidence that CS during MT has advantages over GA in terms of complications, time intervals, and functional outcome.
Introduction
While mechanical thrombectomy (MT) is the standard of care in eligible patients with large vessel occlusion (LVO), the appropriate anesthesia regimen is still an unresolved issue. The primary goal of sedation management during MT is to enable the interventionalist to perform the procedure as quickly and safely as possible. However, studies on this topic have shown conflicting results. A recent meta-analysis from three randomized single-center trials showed an advantage for general anesthesia (GA) compared to conscious sedation (CS) in patients with anterior circulation strokes who underwent MT with respect to 3-month outcome [1]. Contrary to this, a post hoc analysis from 797 patients from the Highly Effective Reperfusion Using Multiple Endovascular Devices (HERMES) Collaboration showed that patients treated without GA had a better 3-month outcome compared to those treated under GA [2]. Data from another registry on anesthesia regimens with 4,429 patients showed [3], that GA was associated with worse functional outcome, especially when compared with local anesthesia. Due to the lack of evidence from large clinical trials on this topic, these results have to be interpreted with caution. The supposed advantages of GA would be the patients’ immobilization during MT, proper pain management, airway protection and therefore fewer periprocedural complications. The more optimal periprocedural conditions are said to lead to higher rates of successful reperfusion resulting in better functional outcome [1]. On the other hand, GA may cause a delay before groin puncture and result in complications related to intensive care management leading to an overall delay in the hospital stay. Advantages of CS include clinical and neurological monitoring, fewer hemodynamic fluctuations, and potentially shorter procedures. However, unprotected airways and patient movement are believed to have a negative influence on functional outcome. Here we aimed to analyze the real-life practice of anesthesia regimen in MT-patients from the German Stroke Registry-Endovascular Treatment (GSR-ET) and its impact on complications and outcome [4].
Methods
Data from the GSR-ET (https://www.clinicaltrials.gov; NCT03356392) were analyzed. Details of the registry have been published previously [4,5]. See Appendix 1 for steering committee members of GSR (GSR investigators).
Study population
Between June 2015 and December 2019, 6,635 patients from 25 sites in Germany with acute ischemic stroke due to LVO, who were treated with MT, were included. The decision for MT was based on the interdisciplinary decision of treating physicians including clinical and imaging parameters and according to national (German Neurological Society) and international guidelines (European Stroke Organization, American Heart Association) [6-8].
Stroke severity was assessed by the National Institutes of Health Stroke Scale (NIHSS). Pre- and post-stroke disability was rated by the modified Rankin Scale (mRS). Early infarct signs were assessed by the Alberta Stroke Program Early CT Score (ASPECTS). Site of occlusion was determined by computed tomography angiography, magnetic resonance angiography, or angiography. Reperfusion success was measured by the modified thrombolysis in cerebral infarction (mTICI) scale and mTICI 2b-3 was rated as successful reperfusion.
The periprocedural anesthesia regimen was based on individual and interdisciplinary decisions and in-house protocols. In general, patients received either GA or CS; a small group converted from CS to GA for various periprocedural reasons. In 313 patients, the anesthesia regimen was not documented.
Statistical analysis
Continuous variables were tested for normal distribution using the Kolmogorov-Smirnov test. Normally distributed data were presented as mean±standard deviation and non-normally distributed data as median (interquartile range) or counts and percentages. Clinical characteristics, imaging data, periprocedural times, and outcome parameters were compared across the three groups using the Kruskal-Wallis test or median-test, as appropriate. Binary logistic regression analysis was performed for good outcome (mRS 0–2) and mortality at follow-up, including variables that were either statistically or clinically (or biologically) significant. For logistic regression analysis, categorical variables were defined as follows: sex male=0, female=1; intravenous thrombolysis treatment no=0, yes=1; successful recanalization no=0, yes=1; type of anesthesia CS=0, GA=1; intracerebral hemorrhage (ICH) no=0, yes=1; tandem lesion no=0, yes=1; posterior circulation stroke no=0, yes =1; pretreatment with oral anticoagulants no=0, yes=1. Converted patients and patients with unknown anesthesia regimen were excluded from the logistic analysis. For all statistical testing, we used the SPSS version 25.0 for Windows (IBM Co., Armonk, NY, USA).
Ethics
The study was conducted in accordance with the Declaration of Helsinki and was centrally approved by the Institutional Review Board (IRB) of the Ludwig-Maximilians-Universität Munich (protocol No. 689-15). Further approval was obtained from local IRBs according to local regulations. Informed consent was not mandatory in accordance with the IRB approval since there were no study-specific procedures performed and data analysis of all patients undergoing MT was demanded by the governmental quality surveillance system.
Results
Out of 6,635 patients from the GSR-ET, 67.1% (n=4,453) underwent GA and 24.9% (n=1,650) CS during MT. In 3.3% of all patients (n=219), the anesthesia regimen was converted from initial CS to GA, while the anesthesia regimen was not documented in 313 patients (4.7%). The CS- and GA-groups were of similar age and sex, but patients from the GA-group had higher pre-stroke modified Rankin Scale (pmRS) and higher NIHSS on admission (Table 1). CS-patients were more often admitted via drip and ship (48.5% vs. 41.2% vs. 49.3%, P<0.001). Further, the CS-group had more often patients with right-sided (48.7% vs. 40.9% vs. 41.1%, P=0.002) and anterior circulation stroke (95.3% vs. 85.0% vs. 88.6%, P<0.001). The rate of successful reperfusion was similar in all three groups (83.0% vs. 84.2% vs. 82.6%, P=0.149). While tandem lesions were more frequent in the GA-group (13.4% vs. 16.4% vs. 17.8%, P=0.011), rate of acute treatment of ipsilateral internal carotid artery (stenting and/or percutaneous transluminal angioplasty) was comparable in all groups (10.2% vs. 11.8% vs. 11.4%, P=0.110). GA compared to CS was associated with a significant delay from admission to groin (61.0 minutes vs. 71.0 minutes vs. 61.0 minutes, P<0.001). Time from groin to flow restoration did not differ between the GA- and the CS-groups (39.0 minutes vs. 41.0 minutes), while the converted group had a significantly longer time interval from groin to flow restoration (64.5 minutes, P<0.001). Overall, the CS-group showed the lowest rate of periprocedural complications (15.0% vs. 21.0% vs. 28.3%, P<0.001).
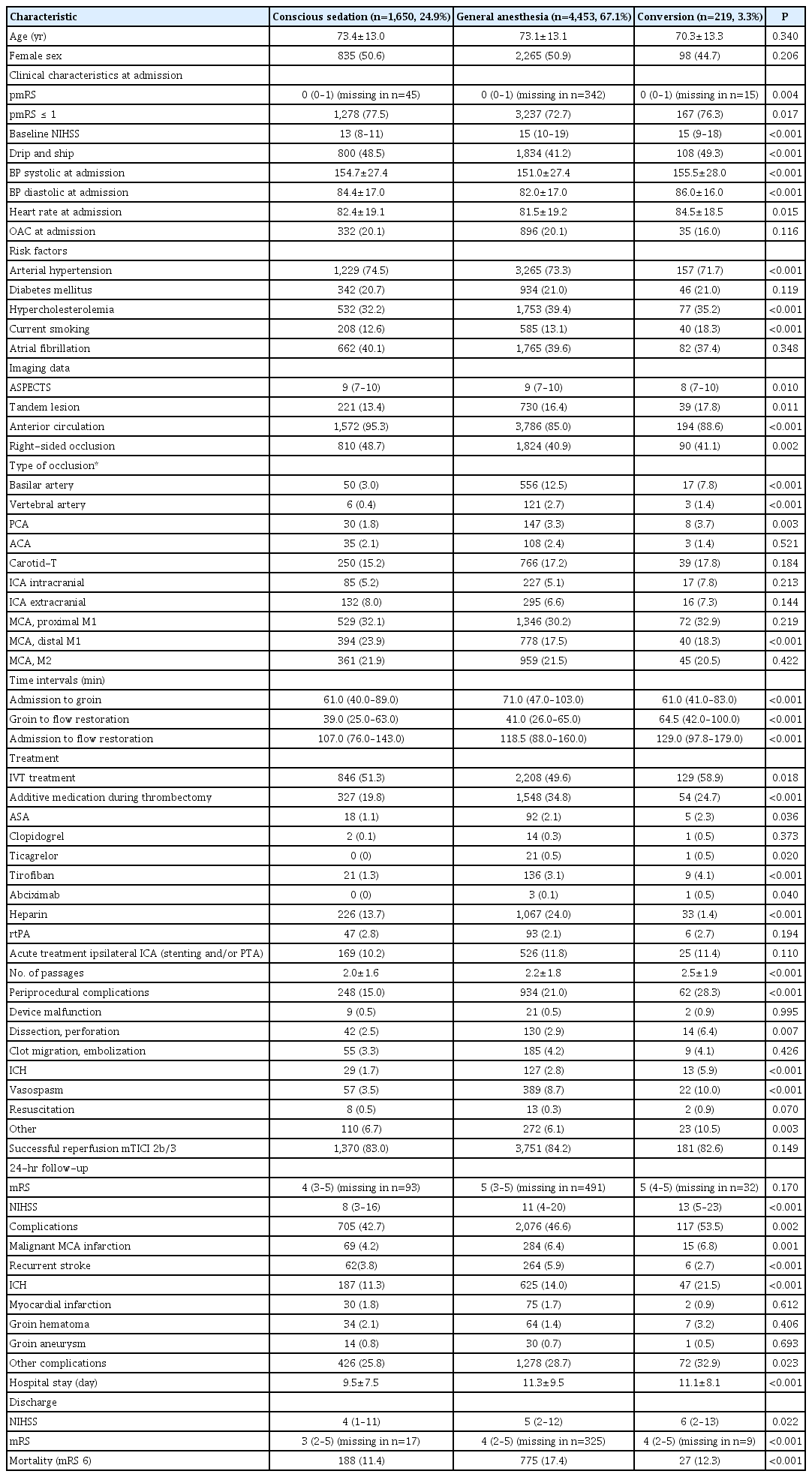
Characteristics of GSR-patients comparing the anesthesia regimen (n=6,635 patients, unknown in n=313)
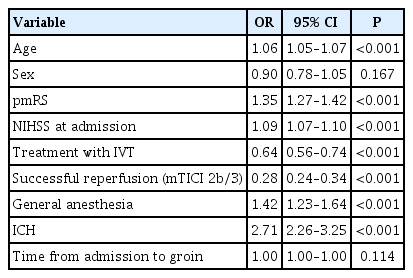
GA was independently associated with higher periprocedural complications (odds ratio [OR], 1.58; 95% confidence interval [CI], 1.35 to 1.86; P<0.001) (Table 2). In the GA-group, patients were more likely to receive intensive antithrombotic medications (Table 1). In the CS-group, rates of periprocedural ICH (1.7% vs. 2.8% vs. 5.9%, P<0.001) and complications during the whole hospital stay (42.7% vs. 46.6% vs. 53.5%, P=0.002) were lowest. Hospital stay was significantly shorter in the CS-group compared to GA-group and the converted group (9.5±7.5 days vs. 11.3±9.5 days vs. 11.1±8.1 days, P<0.001).
Logistic regression for periprocedural complications during mechanical thrombectomy in general anesthesia- and conscious sedation-groups
At 24 hours, CS patients performed better with respect to NIHSS (8 vs. 11 vs. 13, P<0.001). Three-month follow up was not available in 15.4% (n=1,021). The patients in the CS-group were more likely to achieve good outcome (42.1% vs. 34.2% vs. 33.5%, P<0.001) and had a lower rate of mortality (23.4% vs. 32.4% vs. 26.0%, P<0.001) (Figure 1A).
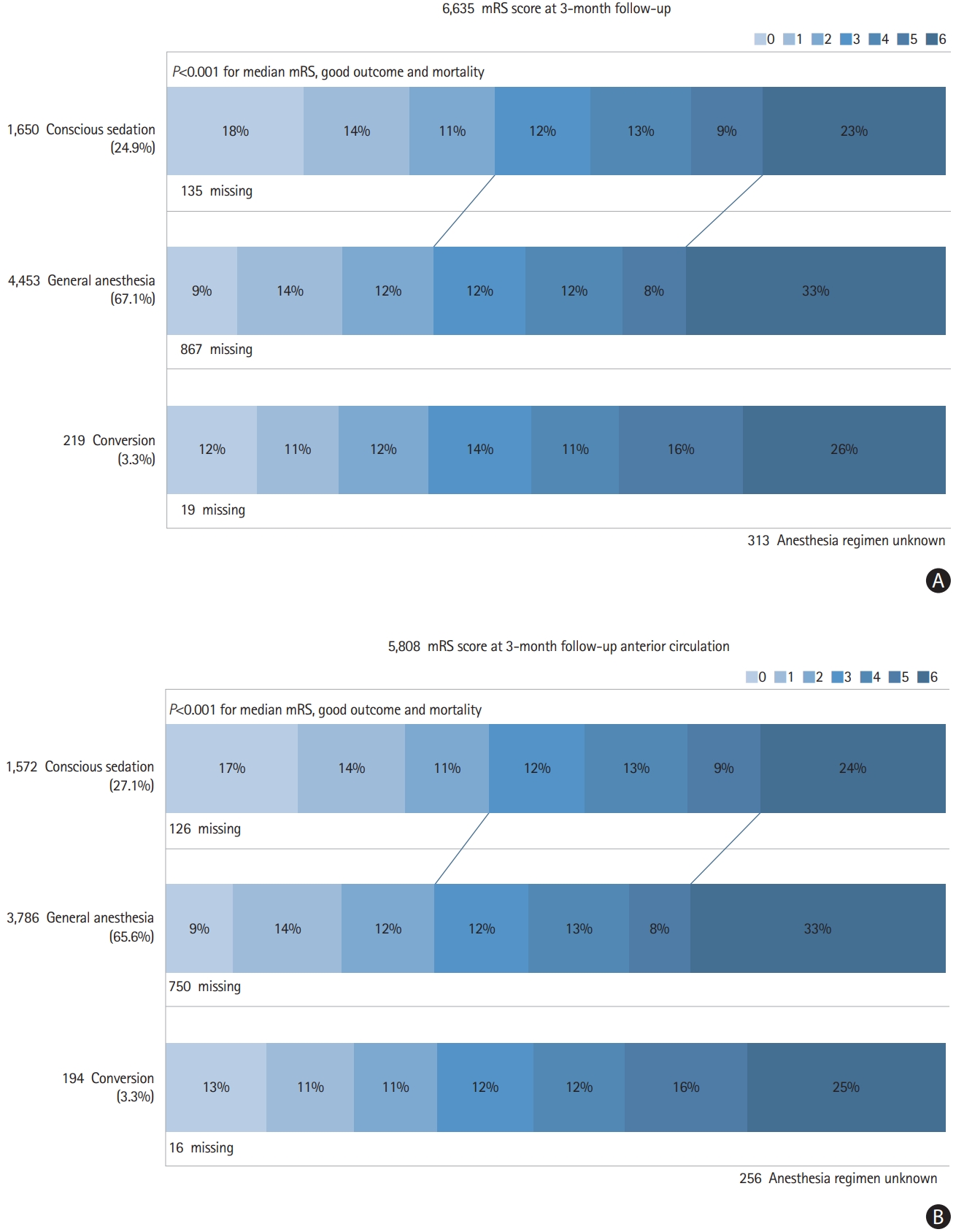
Modified Rankin Scale (mRS) at 3-months follow-up for all large vessel occlusion stroke (A) and for anterior circulation stroke (B). Good outcome mRS ≤2.
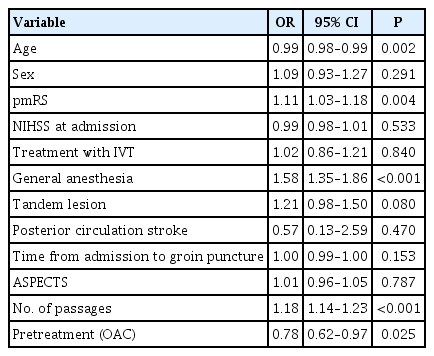
In multivariable analysis, GA was associated with reduced achievement of good functional outcome (OR, 0.82; 95% CI, 0.71 to 0.94; P=0.004) and increased mortality (OR, 1.42; 95% CI, 1.23 to 1.64; P<0.001) (Tables 3 and 4).
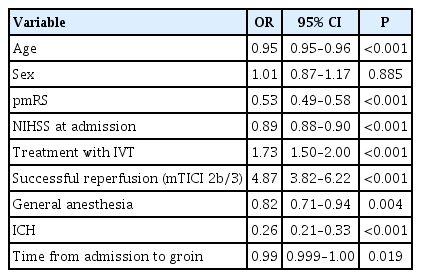
Logistic regression for good outcome in general anesthesia- and conscious sedation-groups at follow-up
We performed an additional analysis, restricted to patients with anterior circulation strokes (n=5,808) (Supplementary Table 1), which showed comparable results with respect to time delay, complications, and outcome parameters (Figure 1B and Supplementary Tables 2 and 3).
Discussion
Here we present by far the largest sample size on anesthesia regimens in 6,635 MT-patients with the following results: GA was the most common strategy in centers within the GSR-ET; more than two-thirds of the patients received MT under GA. The GA-rate is considerably higher than reported in data from clinical trials or registry data with about 30% of patients receiving GA (28.0% to 37.6%) [9-12]. In about 12% of patients starting MT under CS, a conversion to GA was necessary (corresponding to 3% of the whole cohort). This is in line with rates that ranged from about 10% to 15% in other studies [13-17]. In our study, patients from the CS-group performed better with respect to clinical outcomes at 24 hours, at discharge, and at follow-up after 3 months than patients with MT under GA.
Time intervals showed delayed start of groin puncture in GA-patients and therefore longer time intervals from admission to flow restoration. Successful reperfusion rate was similar across all three patient groups. More periprocedural and in-hospital complications were observed under GA—independently of possible confounders, such as tandem lesions, posterior circulation stroke, or number of passages. The interpretation of this result remains speculative. Possibly, there are confounders we cannot address appropriately yet, such as changes in blood pressure caused by GA or imbalances in baseline parameters. Thus the regression analysis cannot appropriately adjust for all these confounders. Well-balanced groups in clinical trials would be necessary to overcome these shortcomings.
Overall, these findings are in contrast to prior studies, but in line with data from another registry analyzing 4,429 patients [3]. A recent meta-analysis from three clinical trials showed an advantage of GA with respect to outcome, an effect that was most probably driven by higher rates of successful reperfusion in the GA-group [1]. Investigators speculated that GA leads to more optimal interventional conditions, resulting in higher reperfusion rates [1]. A post hoc analysis from 797 patients from the HERMES collaboration showed non-GA patients to have a higher rate of good outcome at 3 months compared to GA-patients [2]. However, in this post hoc analysis only 30% of patients were treated under GA. Besides, they used data from RCTs, which were much more homogenous with respect to pmRS, baseline parameters, and occlusion side than in our study [2]. Poorer outcome in the GA-group was most probably not only caused by periprocedural complications but could partly be explained by the fact that the GA- and CS-groups were not fully balanced. With respect to periprocedural complications, we observed higher rates of dissection and perforation, ICH, and vasospasm in the GA-group and the converted group. These complications could have been clinical reason for conversion from CS to GA.
A recent analysis from a prospective Italian registry (Italian Registry of Endovascular Treatment of Acute Stroke [IRETAS]) showed that two-thirds of these patients were treated with CS or local anesthesia. In contrast, in centers from the GSR-ET two thirds of patients underwent MT under GA. Our patients had a pmRS of 0–1 in about 80% compared to up to 90% in IRETAS. ASPECTS in the GSR was 8–9 and 10 in IRETAS. This might partially explain the observation of different sedation regimens. However, due to different sedation regimens and different study populations the comparability of both studies is limited. Besides, our data provide further evidence for anesthesia and sedation management, especially regarding periprocedural complications that have been less well explored using a large dataset.
Our data show significantly lower systolic and diastolic blood pressure and heart rate at admission in the GA-group. However, we have no data on the temporal changes of blood pressure data, especially a significant drop which potentially increases infarct size, leading to poor outcome [18]. In the GA-group, the cumulative dose of norepinephrine—reflecting the drop in blood pressure under GA—was an independent predictor of an unfavorable outcome [19]. On the other hand, vasospasm occurred most frequently under GA. As high PaO2 is discussed to cause intracerebral vasospasm, this effect might be more likely to occur under GA than under CS [20]. However, our data do not allow any conclusion on causality to be drawn. Thus, the causality between GA and higher occurrence of vasospasm as a periprocedural complication is speculative. In converted patients, we neither provided information on the time of the periprocedural complication nor of the conversion from GA to CS. Therefore, we cannot conclude that the periprocedural complication rate is lower in the GA-group.
With regard to the total complication rate during the hospital stay, there were clear differences with a disadvantage of GA, especially regarding the occurrence of ICH and all other complications. In the GA-group, antithrombotic medication was used more intensively. In terms of complications, rates of ICH were highest in patients treated under GA. In summary, patients under GA performed worse in every aspect.
We observed no influence of anesthesia regimen on successful reperfusion rates. The number of passages was highest in the converted group and lowest in the CS-group. However, in the GA-group, periprocedural time to groin puncture was extended by an average of 10 minutes and the delay was not compensated by the MT procedure time. Retrospective studies have suggested that the time delay through intubation may lead to worse outcome [21]. While patients undergoing MT under CS had slightly lower NIHSS at admission (13 vs. 15 in median), the difference in clinical stroke severity continued and even increased with respect to NIHSS and mRS after 24 hours, at discharge, and at 3-month follow-up. Regression analysis showed that CS was independently associated with higher probability of good outcome and lower odds for mortality at 3 months adjusted to confounders like baseline NIHSS. Patients who converted from CS to GA also had better outcome than patients in the GA- group. Besides poorer clinical outcome, GA was associated with a longer hospital stay of 2 days.
The strengths of our study include the large study cohort prospectively collected from a nationwide multicenter registry. Our data therefore represent clinical decisions on sedation management and anesthesia regimen in daily life practice, outside clinical trials. We observed the same results for the whole cohort of LVO patients as well as for anterior circulation strokes. We were able to analyze detailed information on complications and clinical follow-up. Given that it is unlikely that evidence will be established from large clinical trials in the near future, our results provide further evidence based on observational data that patients benefit from CS compared to GA.
However, our study has some limitations. Our data are observational, non-randomized and non-controlled. Therefore, interpretation of the data is prone to bias. Underlying selection bias for decision for an anesthesia regimen have to be discussed as well, even if individual variables in the patient baseline data did not differ. Individual clinical parameters, such as pmRS, NIHSS at admission or presence of tandem lesions, could have contributed to the decision for anesthesia regimens; however, we cannot analyze this in more detail retrospectively. Furthermore, we provide no information about the periprocedural use of sedative agents (type, dosage), further need of intensive care treatment, the duration of mechanical ventilation, and the reason for conversion from GA to CS. Apart from the vital signs at admission, there are no extensive data on the course of these vital signs and their impact on complications and outcome. Further, the CS-group also included patients who only received local anesthesia as a distinction between local anesthesia and CS was not made in the GSR-ET register.
Conclusions
Our study showed an influence on the functional and clinical outcome after MT by the choice of anesthesia regimen with advantages for CS compared to GA in terms of complications, functional outcome, and mortality.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2020.02404.
Characteristics of GSR patients with anterior circulation stroke comparing the anesthesia regimen (n=5,808 patients, unknown in n=256)
Logistic regression for good outcome in anterior circulation stroke patients at follow-up
Logistic regression for mortality in anterior circulation stroke patients at follow-up
Notes
Disclosure
Thomas Liebig consults for Stryker Neurovascular GmbH and has received speaker honoraria from Pfizer, Covidien, Phenox, and Microvention outside this study.
Lars Kellert has received funding for travel or speaker honoraria from Bayer Vital, Boehringer Ingelheim, Bristol-Meyer-Squibb, Daiichi Sankyo, and Pfizer outside of this study.
Acknowledgements
The authors thank Katie Göttlinger and J Stuart Lund for proofreading the manuscript.