Introduction
Stroke is the second most frequent cause of death worldwide [1]. In China, there is 2.5 million new stroke cases annually and 7.5 million stroke survivors [2]. Therefore, it is important to explore more efficient and targeted prevention measures to reduce the stroke incidence.
One convenient way to determine the individuals with potential risks for stroke is to collect family history information. Regularly updating family histories was recommended by the 2002 American Heart Association guidelines for primary prevention of cardiovascular disease [3]. A series of cohort studies have consistently showed the association of family history of stroke (FHS) with increased risk of stroke, but the effects of FHS varied with the different studies [4-13]. This may partly be due to the population discrepancy and small sample size (the largest one including 53,691 participants [4]). To date, only one Chinese cohort study with 15,131 participants and one Japanese cohort study with 53,691 individuals focused on this association in Asians [4,13].
The differences have been reported between different types of FHS (maternal, paternal, and sibling history) in relationship with incident stroke [7,14-18]. Sibling history was more strongly correlated with incident stroke than parental history [14], although the difference was not always observed [15]. The risk of incident stroke was also different in individuals with maternal history versus paternal history [7,16-19]. However, the cohort study is still rare to investigate the contributions of different types of FHS to stroke, especially in Chinese population.
Therefore, we intend to systematically evaluate the relationship between FHS and incident stroke in a large-scale, Chinese population-based cohort study.
Methods
Study population
This study was based on the China Kadoorie Biobank (CKB, known previously as the Kadoorie Study of Chronic Disease in China [KSCDC]). The study rationale, design, survey methods, and baseline population characteristics of the CKB study have been previously described in detail [20,21]. In brief, 512,891 participants aged 30-79 years were enrolled between 2004 and 2008 in 10 regions of China. In this study, we excluded 2,577 persons with cancer, 15,472 persons with heart disease, and 8,884 persons with stroke at baseline. In addition, 14,543 individuals with no information about FHS (maternal, paternal or sibling history of stroke) at baseline were also excluded. The final analyses included 473,849 individuals (192,834 men and 281,015 women).
Ethical approval for our study was obtained from Central Ethical Committee of the Chinese Center for Disease Control and Prevention (Beijing, China), and the Oxford Tropical Research Ethics Committee, the University of Oxford (UK). All participants provided written informed consent.
Ascertainment of outcomes
The end point during the follow-up was incident stroke, including mortality and morbidity data. The vital status of study participants was ascertained through linkage with local disease surveillance points system death certificates and official residential records. Any deaths occurred were coded by trained staff “blinded” to baseline information. Causes of death provided by official death certificates were supplemented, if necessary, separate active confirmation could be carried out by reviewing medical records, reviewing residential records, visiting local communities, or directly contacting participants. Besides, linkage to a local health insurance database was also an important supplementary way of ascertaining deaths. For any additional deaths not verified by routine procedures, the causes could be identified through reviewing hospital records or conducting a verbal autopsy. Information on non-fatal stroke was collected by means of linkage with the established disease registries, as well as the national health insurance claim databases. In addition, participants who failed to be included in the health insurance system were followed annually by trained staff to ascertain their status including hospital admission, disease development, migration and death.
Incident strokes were coded as ischemic stroke (I63), hemorrhagic stroke (subarachnoid [I60] or intracerebral [I61]) and other or unknown stroke type (I64) by trained staff “blinded” to baseline information (using the 10th International Classification of Diseases, ICD-10). Besides, stroke cases were further reviewed by a group of professional neurologists according to uniform and standardized diagnostic criteria. Follow-up time was calculated for each participant from the date of the start of study until the date of the occurrence of stroke, loss to follow-up, or the end of follow-up (December 31, 2013), whichever occurred first.
Assessment of FHS
In the baseline questionnaire, each subject was asked by the interviewer whether the family member (father, mother, or siblings) had been affected by stroke. For siblings, the number of affected members with stroke was recorded. We defined a participant as “family history positive” if he or she reported that one parent or sibling had stroke. Positive parental history was defined as reporting a positive history for either or both parents. In this study, first degree relatives included fathers, mothers, and siblings. For each subject, the first degree family members’ stroke history had been collected.
Assessment of covariates
All participants completed the standardized questionnaires covering detailed questions on general socioeconomic and demographic status, health status and medical history (hypertension, diabetes, stroke or transient ischemic attack, cancer and heart disease), smoking status, alcohol consumption, and other lifestyle behaviors. Variables involving general socioeconomic and demographic status included age, sex, residential area, occupation, etc. The level of physical activity was calculated as metabolic equivalent task hours daily (metabolic equivalent of task-hours/day). Besides, the number of siblings was covered.
Physical measurements were made by trained staff using calibrated instruments including body height, weight, waist and hip circumference, heart rate and blood pressure. Body mass index was calculated as weight (kg) divided by the square of height (m2). Prevalent diabetes was defined as measured fasting blood glucose 37.0 mmol/L, measured random blood glucose 311.1 mmol/L, or self-reported diagnosis of diabetes. Prevalent hypertension was defined as measured systolic blood pressure 3,140 mm Hg, measured diastolic blood pressure 390 mm Hg, self-reported diagnosis of hypertension, or self-reported use of antihypertensive agents at baseline.
Statistical analyses
Continuous data were presented as mean (standard deviation) and categorical variables as counts and frequencies. We compared means by the method of Student’s t-test, and categorical variables were compared using Pearson’s χ2 statistic to determine differences in the baseline characteristics between subjects with and without FHS. The Cox proportional hazards regression model was used to evaluate hazard ratios (HRs) and 95% confidence intervals (CIs) of stroke for FHS. We also estimated the HRs of stroke according to the status of FHS (without FHS, with FHS, with 1, with 2, or with ≥3 ill family members). Besides, the HRs were evaluated according to types of FHS (including father, mother, and sibling history). Analyses were done for all incident stroke and also separately for each stroke type. The multivariate adjusted HRs of stroke were estimated with the following adjustments: age (continuous); sex (male or female); residential area (rural or urban); level of education (no formal school, primary school, middle school, high school, technical school/college, or university); marital status (married, widowed, divorced/separated, or never married); alcohol consumption (non-drinker, occasional drinker, ex-drinker, or current regular drinker); smoking status (never smoker, occasional smoker, ex-smoker, or current regular smoker); physical activity (continuous); body mass index (BMI) (continuous); prevalent hypertension and diabetes at baseline (presence or absence) and number of siblings (continuous).
Stratified analyses were carried on: age (<45, 45 to 54, 55 to 64, or 365 yr), gender (male or female), marital status (married or widowed/separated/divorced/never married), residential area (rural or urban), education (Illiteracy/primary school or middle school and above), smoking status (current regular smoker or not), alcohol consumption (current regular drinker or not), physical activity (categorized using median), BMI (<24.0, 24.0 to 27.9, or 328.0), and prevalent hypertension and diabetes at baseline (presence or absence). The interaction analyses were performed by means of likelihood-ratio tests, comparing models with and without the interaction items between the baseline stratifying variables and FHS as a dichotomous variable.
All CIs were estimated at the 95% level. All statistical tests were two-sided and significance was defined as P<0.05. The statistical analyses were performed with R software (version 3.1.1, 2014-07-10; R Foundation for Statistical Computing, http://www.cran.r-project.org/).
Results
Baseline characteristics of the study population were summarized in Table 1. The proportion of participants with FHS was 18.15%. Compared with participants without FHS, those with FHS were more likely to be men, older, in urban areas, currently married, highly educated, drink and smoke, and more likely to have a higher BMI, lower physical activity, and hypertension or diabetes problem.
Total person-years of follow-up were 3,338,261 and the median follow-up time was 7.16 years. A total of 26,395 (5.57%) stroke cases were reported (20,528 of ischemic stroke, 4,968 of hemorrhagic stroke and 899 of other conditions). Incidence rate of stroke per 1,000 person years was 7.31 for participants without FHS, and 10.62 for participants with FHS. Incidence rates of stroke according to the number of family members with stroke were 9.78, 15.44, and 24.29 per 1,000 person years for subjects whose FHS involving 1, 2, and ≥3 members, respectively (Table 2). The trend that the incidence of stroke increased with the number of the first degree relatives with stroke was similar for both hemorrhagic and ischemic stroke.
The age- and gender- adjusted HR (95% CI) of stroke for participants with FHS was 1.50 (1.46-1.55) as compared with those without FHS (Table 2). According to the number of family members with stroke, the age- and gender- adjusted HRs (95% CIs) of stroke were 1.41 (1.37-1.46), 1.98 (1.86-2.11), and 2.47 (2.15-2.84) for subjects whose FHS involving 1, 2, and ≥3 members (Ptrend <0.001), respectively, in comparison with those without FHS. The HRs increased with the increasing number of first degree relatives with stroke and this trend remained after multivariable adjustment (Ptrend <0.001). In Table 2, the HRs for hemorrhagic stroke were increased with the number of first degree relatives with stroke, which also was the case for ischemic stroke.
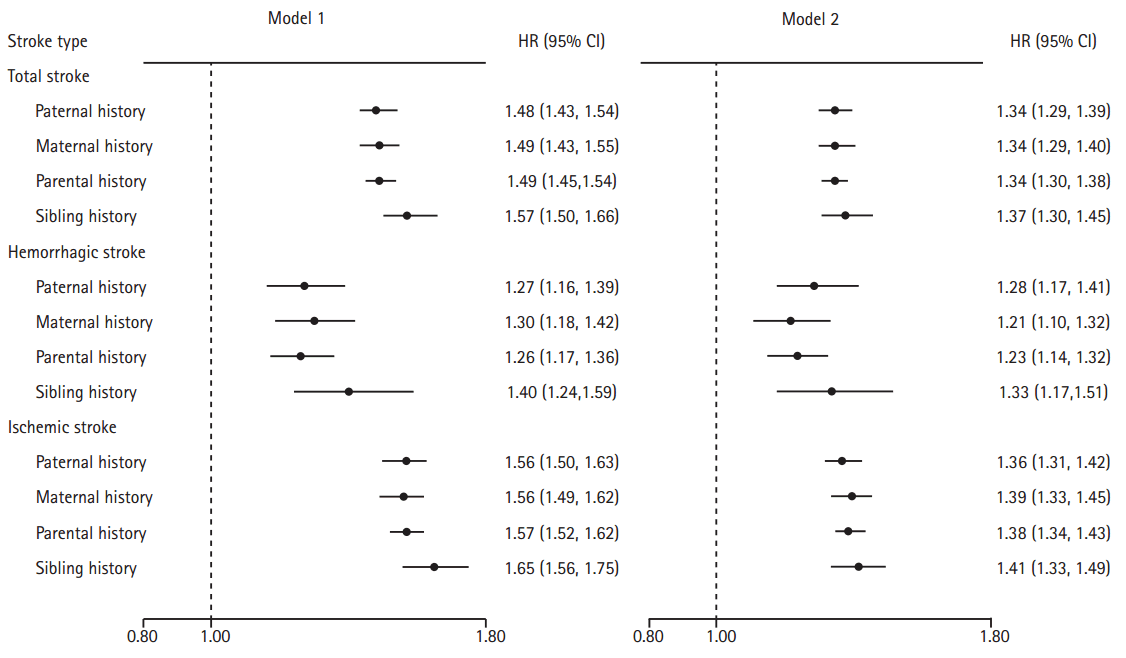
The age- and gender- adjusted HRs were 1.57 (95% CI: 1.50-1.66) and 1.49 (95% CI: 1.45-1.54) for sibling history and parental history, respectively, and the difference was not statistically significant (Pheterogeneity=0.072) (Figure 1). After the multivariable adjustment, the HR (95% CI) was 1.37 (1.30-1.45) for sibling history and 1.34 (1.30-1.38) for parental history (Pheterogeneity=0.434). Age- and gender- adjusted HRs (95% CIs) related to incident stroke were similar between individuals with paternal history (1.48 [1.43-1.54]) and maternal history (1.49 [1.43-1.55]) (Pheterogeneity=0.866). After multivariable adjustment, the HRs and 95% CIs of incident stroke relevant to paternal history (1.34 [1.29-1.39]) and maternal history (1.34 [1.29-1.40]) remained comparable (Pheterogeneity=0.846). Moreover, men and women had similar risk of incident stroke whenever their father or mother had stroke (all Pheterogeneity>0.05 between men and women in the associations of each type of FHS) (Supplementary Table 1). Similar patterns were also shown for both hemorrhagic and ischemic stroke in terms of the difference between sibling and parental history and in terms of the consistence between paternal and maternal history (Figure 1, Supplementary Table 1).
We further performed stratified analyses according to age groups (classified as <45, 45-54, 55-64, and ≥65 yr). We found that the HRs of incident stroke related to FHS decreased with age, and the HRs (95% CIs) were 1.60 (1.46-1.76), 1.51 (1.43-1.59), 1.25 (1.19-1.31), and 1.14 (1.08-1.21), respectively (Figure 2). This trend was also obvious in both hemorrhagic and ischemic stroke in Figure 2. In the stratification analyses based on the other factors, individuals with FHS were more likely to be affected by stroke if they were currently tobacco smoking or alcohol drinking (P<0.05 after Bonferroni correction for heterogeneity test between subgroups) (Supplementary Table 2). Further analyses revealed significant interactions between these two health risk behaviors and FHS on the risk of stroke (Supplementary Table 2). Particularly, we observed a synergistic effect of FHS and current regular smoking on the risk of stroke regardless of types of stroke (Supplementary Figure 1).
Discussion
This Chinese cohort study showed that FHS was an independent risk factor for developing stroke, for whatever type of FHS. The more first degree relatives are affected by stroke, the higher are individuals’ risk of suffering from stroke. Maternal history of stroke and paternal history of stroke had no differential effects on the risk of stroke, in both men and women. Individuals reported a positive FHS at young age had higher risk of stoke than those at late age. Moreover, FHS interacted with the health risk behaviors (smoking status and alcohol consumption) on the risk of stroke occurrence.
FHS represents a combination of genetic factors and shared environmental factors. It has been reported that compared with parent-offspring pairs, shared environmental effects on cardiovascular risk factors were stronger within sibling pairs [22,23]. In addition, some studies reported that sibling history was more strongly correlated with the development and severity of stroke than parental history [14,24]. Although the difference was not statistically significant, we found that sibling history was more strongly associated with stroke as opposed to parental history according to the purely numerical values. Additionally, this difference of purely numerical values between sibling history and parental history decreased after further adjusting for other covariates, including education, residential area, smoking and drinking status, which might highlight the contribution of shared environment to the sibling history of stroke.
Previous studies provided evidence for that the relationship between maternal history and stroke was different from that between paternal history and stroke [7,16-18]. Touzé and Rothwell showed that the relationship between maternal and offspring stroke was stronger in females relative to males (sample size: 806) [16], and this result was validated by a meta-analysis (total sample size: 7,941) [19]. However, Seshadri et al. reported that there was a marginally stronger association of paternal stroke with offspring stroke in comparison with maternal stroke (sample size: 3,443) [7]. Different from the above mentioned studies in Caucasian population, we firstly compared the differences of heritability of stroke between paternal and maternal history in this Chinese cohort study of 0.5 million individuals, and observed the same HRs for these two types of family history for both males and females. Thus, maternal history and paternal history need our equal attention in assessing heritability of stroke in Chinese population regardless of gender.
Numerous factors have been identified to be implicated with stroke occurrence in epidemiological research [25]. In agreement with other studies [26,27], our study revealed that young age was more strongly associated with FHS in comparison with old age, which was consistent in both hemorrhagic stroke and ischemic stroke. A probable interpretation is that the relative importance of FHS and genetic factors may decrease with advancing age and other postnatal factors may become more prominent [28]. In addition, we found significant interactions between FHS and two health-risk behaviors, including smoking and alcohol consumption. Particularly, we identified a synergistic effect of FHS and smoking on the risk of stroke regardless of types of stroke. Smoking was one of the major risk factors of stroke and might affect the concentration of high density lipoprotein, increase blood fibrinogen levels, promote atherosclerosis formation, and further increase the risk of hypertension and diabetes (modifiable risk factors of stroke) [29,30]. Thus, smoking would increase the incidence risk of stroke in Chinese population, especially for the individuals with FHS, which emphasizing the importance of management of smoking. Similarly, alcohol drinking is also related to stroke risk, especially for the individuals with FHS. Therefore, the importance of management of the health risk behaviors for reducing stroke risk should be highlighted for Chinese population, especially for the individuals with FHS.
Strengths of our study are the prospective cohort design, the large sample size, the well-designed questionnaire, and the efficient data collection and management. There are also some limitations that need to be mentioned. First, we did not obtain the information about the age of onset of parental stroke. Thus, we could not explore the association of early-onset stroke in parents to incident stroke in offspring. Second, family history data were obtained based on self-report, which might affect the accuracy of the information and lead to some degree of misclassification bias. However, in self-reported surveys, the recall bias is inevitable, and in comparison with case-control studies, the recall bias was less in our cohort study. Besides, previous studies have reported that self-reported history is reliable for stroke data [31]. Third, some stroke risk factors were not adjusted for (such as serum total cholesterol) because the relevant information was not collected. These factors may partly explain the stroke risk associated with FHS.
Conclusions
FHS is an independent risk factor for stroke in Chinese, and the more first degree relatives are affected by stroke, the higher are individuals’ risk of suffering from stroke. The management of the health risk behaviors for reducing stroke should be highlighted, especially for the individuals with FHS.