|
 |
- Search
| J Stroke > Volume 23(2); 2021 > Article |
|
Dear Sir:
Recent studies, including our previous investigation, indicate that cerebral microbleeds (CMBs) are significantly associated with a higher risk of hemorrhagic stroke among patients taking oral anticoagulants (OACs) [1,2]. Physicians, therefore, are confronted with a dilemma regarding the proper administration of antithrombotic therapy to patients with both atrial fibrillation (AF) and CMB due to the patients’ competing ischemic and hemorrhagic conditions [3]. This dilemma may be especially pronounced in patients with severe CMB patterns (multiple or lobar CMBs) [3]. Nevertheless, no study had directly compared the effectiveness of administering guideline-recommended OACs with that of antiplatelet therapy, which is used in a large portion of patients with AF in real-world practice, for preventing recurrent stroke in patients with both AF and CMB [4]. Therefore, we investigated the efficacy and safety of OACs versus those of antiplatelet therapy in reducing the future risk of stroke in these patients.
This study is a retrospective analysis of a prospective registry. The primary outcome measurement was the incidence of recurrent stroke (ischemic or hemorrhagic) at any time during the final 2 years of follow-up. We used propensity scores and stabilized inverse probability of treatment weighting (IPTW) to balance possible confounders. Detailed methodical descriptions are provided in the Supplementary Methods. This study included 483 patients with CMB and acute ischemic stroke associated with AF (Supplementary Figure 1). The clinical and biochemical characteristics of the patients before and after IPTW are shown in Supplementary Table 1. Compared with those in the OAC group, the patients in the antiplatelet group were more likely to have severe initial symptoms, cerebral atherosclerosis, and a history of diabetes mellitus. Absolute standardized differences were balanced after propensity score matching (Supplementary Figure 2).
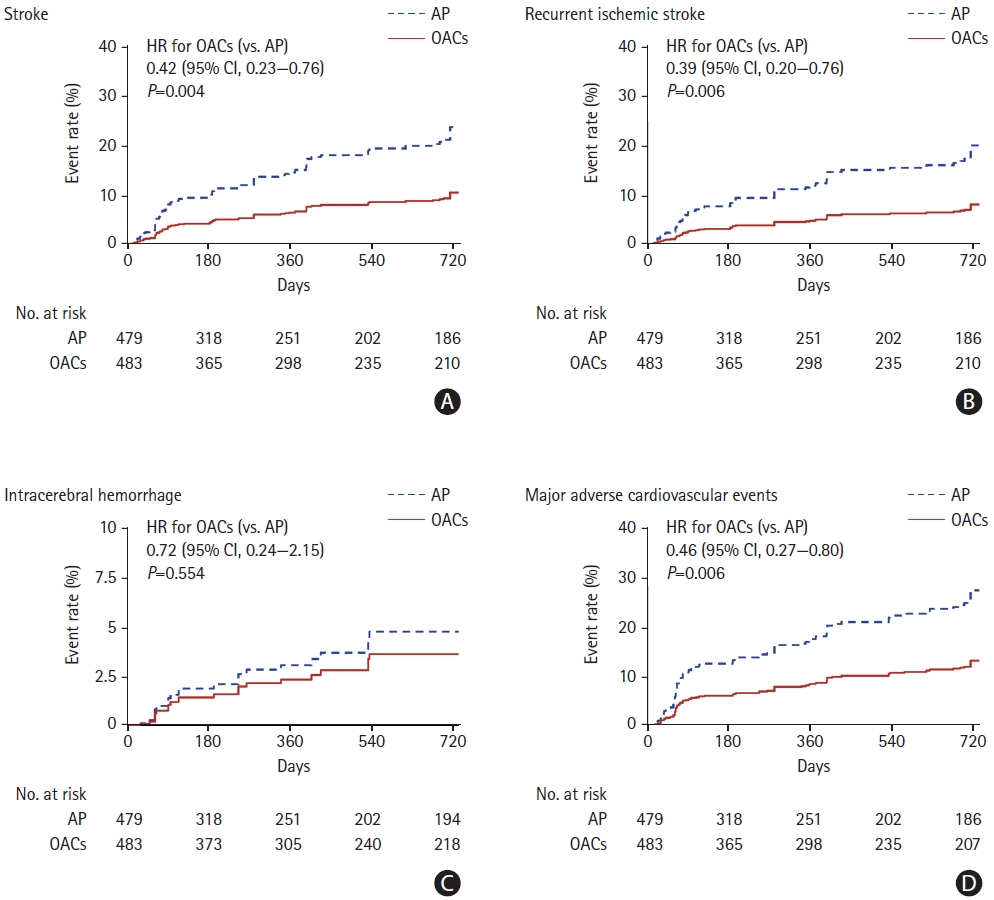
We found that 51 patients experienced a stroke during follow- up. Patients treated with OACs had a significantly lower risk of future stroke (hazard ratio [HR], 0.42; 95% confidence interval [CI], 0.23 to 0.76; P=0.004) (Figure 1 and Supplementary Table 2) than did the patients who were treated with antiplatelet therapy. In particular, the non-vitamin K antagonist OACs group had a significantly lower risk of recurrent stroke than did the antiplatelet group (HR, 0.22; 95% CI, 0.06 to 0.74; P=0.014) (Supplementary Table 2). Regarding key secondary outcomes, the OAC group had a significantly lower rate of recurrent ischemic stroke, all-cause death, and major adverse cardiovascular events relative to the antiplatelet group. By contrast, the rates of intracerebral hemorrhage were similar between the antiplatelet and OAC groups (Figure 1 and Supplementary Table 2).
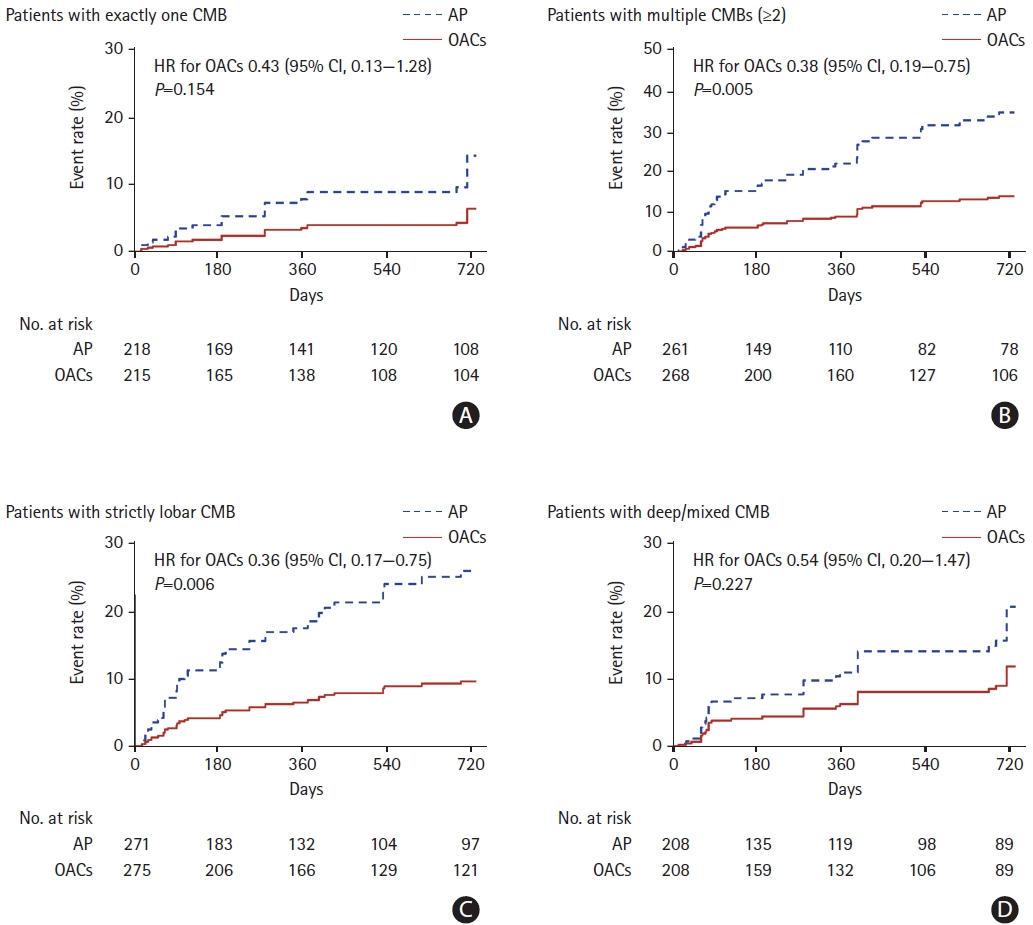
Among the patients with exactly one CMB, the rate of stroke was lower in the OAC group than in the antiplatelet group; however, this difference was nonsignificant (HR, 0.43; 95% CI, 0.13 to 1.28; P=0.154) (Figure 2 and Supplementary Table 2). By contrast, OACs were significantly associated with a reduced risk of stroke in patients with multiple CMBs (HR, 0.38; 95% CI, 0.19 to 0.75; P=0.005) compared to antiplatelet therapy. Among patients with strictly lobar CMB, the risk of stroke was also significantly lower in the OAC group than in the antiplatelet group (HR, 0.36; 95% CI, 0.17 to 0.75; P=0.006). However, there was no significant difference in the risk of stroke between the OAC and antiplatelet groups among patients with deep/mixed CMB (Figure 2 and Supplementary Table 2).
Our study suggests that the administration of OACs to ischemic stroke patients with AF and CMB is significantly associated with a lower risk of future stroke than antiplatelet therapy. Despite the common concern that OACs may cause intracerebral hemorrhage in patients with severe CMB patterns, our study shows that patients with multiple or lobar CMBs may benefit more from OACs than antiplatelet therapy. The higher risk of ischemic stroke than hemorrhagic stroke among patients with CMB may account for this finding [1,5]; the crude incidence of ischemic stroke was more than twofold that of hemorrhagic stroke in our population, even among patients with severe CMB patterns. As the absolute risk of ischemic stroke in patients with CMBs can increase as the CMB burden increases [6], choosing to administer OACs rather than antiplatelet therapy would be beneficial to patients with multiple CMBs. The only study to date comparing the effects of OACs with those of antiplatelet therapy in ischemic stroke patients with AF and CMB estimated that patients with greater CMB counts might receive a greater benefit from OACs than from aspirin therapy [5]. However, the estimates of the study were calculated indirectly from the data reported by previous studies. Our real-world study supports previous estimates concerning the benefit of OACs compared to antiplatelet therapy in the prevention of future stroke among ischemic stroke patients with AF and CMB.
Our study is subject to limitations. As an observational, retrospective analysis of a relatively small sample, this investigation has methodological shortcoming. In addition, our data are prone to the influences of potential selection bias and unmeasured confounders. However, to reduce the risk of confounding, we used propensity score analyses.
In conclusion, our results indicate that for the secondary prevention of stroke in ischemic stroke patients with AF and CMB, the clinical benefits of guideline-recommended OACs can outweigh the risks in comparison to antiplatelet therapy. The net benefits of OAC therapy may be particularly pronounced in patients with multiple or lobar CMBs. Further studies are required to better characterize and tailor optimal antithrombotic treatment for ischemic stroke patients with AF and CMB.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2020.04588.
Supplementary Table 1.
Baseline characteristics according to antiplatelet treatment before and after IPTW
Supplementary Table 2.
Efficacy and safety of oral anticoagulant and antiplatelet therapies
Supplementary Figure 1.
Subject enrollment flowchart. MRI, magnetic resonance imaging.
Supplementary Figure 2.
Absolute standardized differences for estimating propensity score before and after inverse probability of treatment weighting (IPTW). TIA, transient ischemic attack; LDL, low-density lipoprotein; HDL, high-density lipoprotein; eGFR, estimated glomerular filtration rate; NIHSS, National Institutes of Health Stroke Scale; mRS, modified Rankin Scale; CHA2DS2-VASc, Congestive heart failure, Hypertension, Age over 75 years (doubled), Diabetes mellitus, Prior Stroke or TIA (doubled), Vascular disease, Age 65 to 74 years, Sex category; HAS-BLED, Hypertension, Abnormal renal and liver function, Stroke, Bleeding, Labile INR, Elderly, Drugs or alcohol; CMB, cerebral microbleed.
Acknowledgments
This work was supported by a National Research Foundation of Korea grant funded by the Korean Government (NRF-2019M3A9E8020261). This study was supported by a grant (HCRI20006) from Chonnam National University Hwasun Hospital Institute for Biomedical Science.
References
1. Wilson D, Ambler G, Shakeshaft C, Brown MM, Charidimou A, Al-Shahi Salman R, et al. Cerebral microbleeds and intracranial haemorrhage risk in patients anticoagulated for atrial fibrillation after acute ischaemic stroke or transient ischaemic attack (CROMIS-2): a multicentre observational cohort study. Lancet Neurol 2018;17:539-547.


2. Choi KH, Kim JH, Lee C, Kim JM, Kang KW, Kim JT, et al. Microbleeds and outcome in patients with acute ischemic stroke and atrial fibrillation taking anticoagulants. Stroke 2020;51:3514-3522.


4. Xian Y, O’Brien EC, Liang L, Xu H, Schwamm LH, Fonarow GC, et al. Association of preceding antithrombotic treatment with acute ischemic stroke severity and in-hospital outcomes among patients with atrial fibrillation. JAMA 2017;317:1057-1067.