Duration of Implantable Cardiac Monitoring and Detection of Atrial Fibrillation in Ischemic Stroke Patients: A Systematic Review and Meta-Analysis
Article information
Abstract
Background and Purpose
Current guidelines do not provide firm directions on atrial fibrillation (AF) screening after ischemic stroke (IS). We sought to investigate the association of implantable cardiac monitoring (ICM) duration with the yield of AF detection in IS patients.
Methods
We included studies reporting AF detection rates by ICM in IS patients with negative initial AF screening. We excluded studies reporting prolonged cardiac monitoring with devices other than ICM, not providing AF detection rates or monitoring duration, and reporting overlapping data for the same population. The random-effects model was used for all pooled estimates and meta-regression analyses.
Results
We included 28 studies (4,531 patients, mean age 65 years). In meta-regression analyses, the proportion of AF detection by ICM was independently associated with monitoring duration (coefficient=0.015; 95% confidence interval [CI], 0.005 to 0.024) and mean patient age (coefficient=0.009; 95% CI, 0.003 to 0.015). No associations were detected with other patient characteristics, including IS subtype (cryptogenic vs. embolic stroke of undetermined source) or time from IS onset to CM implantation. In subgroup analyses, significant differences (P<0.001) in the AF detection rates were found for ICM duration (<6 months: 5% [95% CI, 3% to 6%]; ≥6 and ≤12 months: 21% [95% CI, 16% to 25%]; >12 and ≤24 months: 26% [95% CI, 22% to 31%]; >24 months: 34% [95% CI, 29% to 39%]).
Conclusions
Extended duration of ICM monitoring and increased patient age are factors that substantially increase AF detection in IS patients with initial negative AF screening.
Introduction
Approximately one-third of all ischemic strokes (IS) are characterized as cryptogenic strokes (CS), due to the lack of a possible cause for the event or incomplete diagnostic work-up [1-3]. Atrial fibrillation (AF), either paroxysmal or chronic, represents a major risk factor for stroke and systemic embolism, and is associated with a 5-fold increase in IS risk [4,5]. Although paroxysmal AF appears to be implicated in at least 30% of patients with CS and in approximately 25% of patients with unselected IS [6,7], current guidelines on secondary stroke prevention do not provide firm directions on AF screening after IS.
The American Heart Association/American Stroke Association (AHA/ASA) recommendations on secondary stroke prevention suggest that prolonged rhythm monitoring for approximately 30 days is reasonable for AF screening within 6 months after CS (Class IIa; Level of Evidence C) [8], while the recent AHA/ASA guidelines on the early management of IS patients indicate that the clinical benefit of prolonged cardiac monitoring to detect AF remains uncertain (Class of Recommendation: IIb, Level of Evidence: B) [9]. However, clinical trials [6,10] suggest that implantable cardiac monitors (ICMs) substantially increase AF detection in IS patients, due to prolonged monitoring duration.
In the present systematic review and meta-analysis, we sought to investigate the association of ICM duration with the level of AF detection in IS patients. We also assessed whether IS subtype, patient characteristics, and elapsed time between IS onset and CM implantation may affect the probability of AF detection.
Methods
Search strategy and selection criteria
This study was conducted according to the Preferred Reporting Items of Systematic Reviews and Meta-Analyses (PRISMA) statement [11]. We searched for studies reporting AF detection rates by ICM in patients with history of IS or transient ischemic attack (TIA). A literature search in MEDLINE, SCOPUS, and the Cochrane Central Register of Controlled Trials (CENTRAL) was performed, using the following terms in combination: “cardiac monitoring,” “implantable loop recorder,” “insertable loop recorder,” “implantable cardiac monitor,” “cryptogenic stroke,” “embolic stroke of undetermined source,” “ischemic stroke,” “cerebral ischemia,” “atrial fibrillation,” and “atrial flutter.” The complete algorithm used in the MEDLINE database search is available in the online Supplementary Methods. Eligible studies were also sourced from a manual search of key journals, conference proceedings and other (non-Cochrane) systematic reviews and meta-analyses. No language or other search restrictions were applied. The last literature search was performed on July 10, 2018.
We included all studies (randomized clinical trials [RCTs], prospective/retrospective cohort studies, case-control studies) reporting detection rates of AF by ICM in patients with history of IS or TIA. We excluded from further evaluation all case reports, case series, studies reporting cardiac monitoring with devices other than ICM, and studies not providing AF detection rate or monitoring duration with ICM. We also excluded studies reporting overlapping population data, and included only the study with the highest number of patients and/or more extended follow-up time. However, we retained publications providing data for distinct IS groups, including CS and embolic stroke of undetermined source (ESUS), despite the possibility of overlapping group data (ESUS overlapping with CS, and CS/ESUS overlapping with unselected IS/TIA). Reference lists of all articles that met the inclusion criteria, and of relevant review articles, were examined to identify studies that may have been missed by the initial database search. All retrieved studies were scanned independently by two reviewers (G.T. and A.H.K.). In case of disagreement regarding the literature search results between the two coauthors, the remaining coauthors were consulted, and disagreement was ultimately resolved with consensus. We used the Newcastle-Ottawa Scale to assess the quality of included studies that were published at the time of the literature search, and to identify potential sources of bias amongst eligible studies [12]. Quality control and bias identification were performed independently by the same authors who performed the literature search (G.T. and A.H.K.), and all potential disagreements were resolved after discussion and mutual consensus.
The minimum required AF duration, for diagnosing AF with ICM, was documented separately for each study protocol. We calculated the ICM AF detection rates for different ICM durations by dividing the number of events (patients with detected AF) by the total number of patients receiving ICM. After the overall analysis we performed meta-regression analyses for all study and patient characteristics that were available in 10 or more of the included studies [13]. We also conducted pre-defined subgroup analyses according to the study type (prospective or retrospective cohort), study population (CS, ESUS, unselected IS/TIA), the specific time threshold used for AF diagnosis (30 seconds, 2 minutes, 6 minutes), the monitoring duration (<6, ≥6 and ≤12, >12 and ≤24, >24 months), the ICM device used, and the elapsed time between IS/TIA onset and implantation of CM (≤1 and >1 month), provided that at least two studies were included in each subgroup. Finally, for all the aforementioned meta-regression and subgroup analyses, we performed additional sensitivity analyses after excluding studies that were presented in conferences and had only abstracts publicly available at the time of the literature search. Data extraction was performed by two independent authors (A.H.K. and L.P.), and in cases of disagreement, a senior author (G.T.) was consulted.
For all proportion analyses we used the variance-stabilizing double arcsine transformation [14]. Pooled estimates in both the overall and subgroup analyses were calculated using the Hartung-Knapp-Sidik-Jonkman method [15]. Meta-regression analyses were performed under the random-effects model (method of moments). Variables with a threshold of P<0.1 in the initial univariate meta-regression analyses were used as covariates for multivariate meta-regression models. Due to the established relationship of age with AF incidence [16], mean age was included as an a priori potential confounder in all multivariate models. The equivalent z test was performed for each pooled estimate and P<0.05 was considered statistically significant. We assessed heterogeneity between studies with the Cochran Q and I2 statistics [17]. For all subgroup analyses we used a standard test for heterogeneity across subgroup results, to investigate for potential differences between subgroups, as previously described [18]. Small-study effect (i.e., publication bias) across individual studies was evaluated graphically using both funnel plot inspection and the Egger’s linear regression test, at a significance level of 0.1 [19].
All statistical analyses were performed using Stata Statistical Software Release version 13 for Windows (StataCorp LP, College Station, TX, USA) and OpenMeta-Analyst software [20].
Since the present work is a systematic review and metaanalysis of previously published studies, IRB approval was waived.
Results
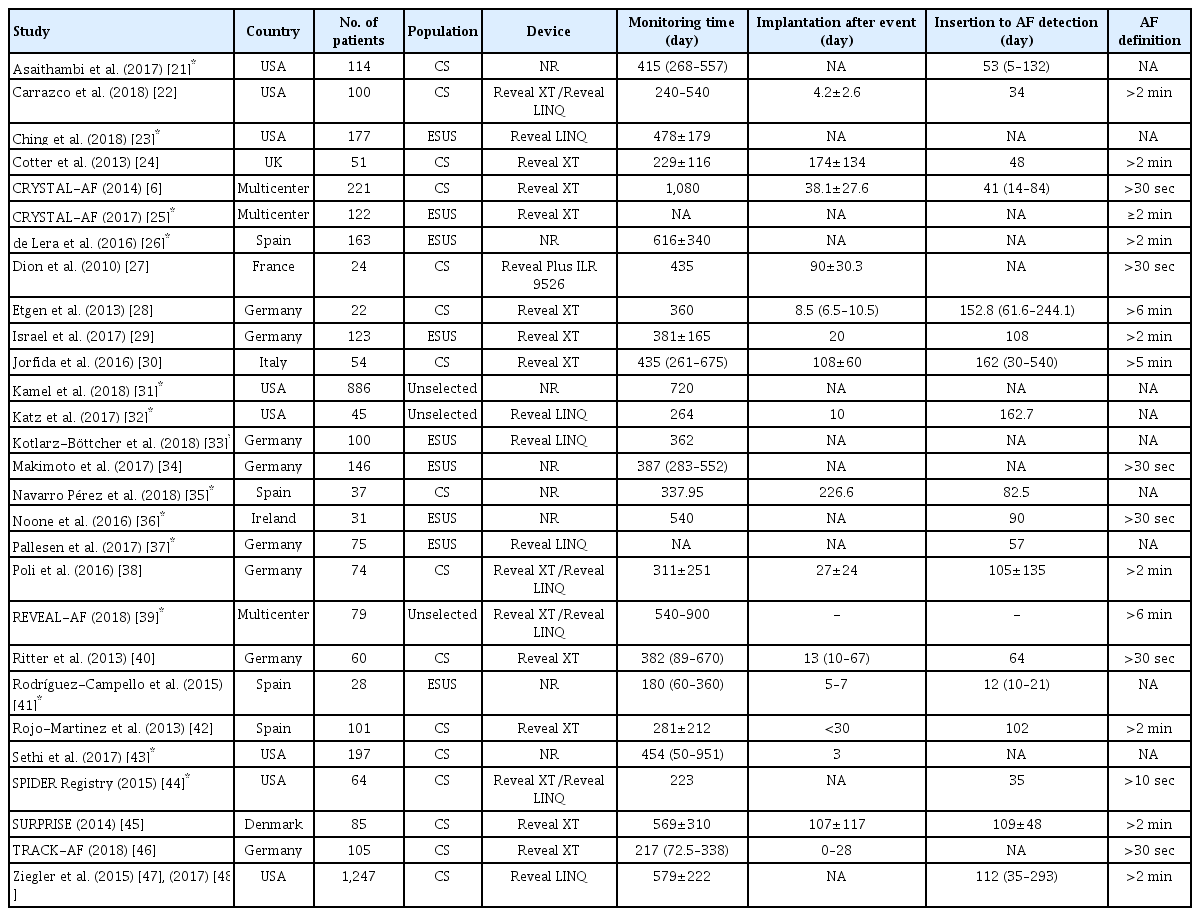
The PRISMA flowchart summarizing the literature search process is shown in Supplementary Figure 1. MEDLINE and SCOPUS literature searches retrieved 375 and 417 results respectively, while comprehensive searches of key journals and conference proceedings identified 20 additional studies. Of all potentially eligible studies, 18 study protocols were excluded (Supplementary Table 1) due to overlapping data (n=4), the use of monitoring devices other than ICM (n=11), or unavailable information on ICM duration (n=3). Our literature search highlighted 28 studies for inclusion, comprising 4,531 patients (mean age 65 years, 52% male) [6,21-48]. Protocols and patient characteristics of included studies are briefly summarized in Table 1 and Supplementary Table 2, respectively. Most studies were conducted in the USA (n=9) and Germany (n=8). The most common subgroup studied was cryptogenic IS/TIA (n=17), followed by ESUS (n=9). The mean/median elapsed time from IS/TIA onset to cardiac monitoring implantation ranged from 3 to 174 days, while the mean/median ICM duration ranged from 180 to 1,080 days (Table 1). Included studies were generally found to have a low risk of bias (Supplementary Table 3), except in cases not clearly stating consecutive enrollment of patients [23,26,27,39,44-47], exclusion of AF with electrocardiogram or short-term non-invasive Holter monitoring prior to ICM impantation [21,33], or no adjudication of ICM recordings by experienced cardiologists [26-29,33,41,46,47].
In the overall analysis of all included studies, the cumulative AF detection rate in patients with ICM was 26% (95% confidence interval [CI], 22% to 30%), with significant heterogeneity among studies (I2=83%, P for Cochran Q <0.001) (Supplementary Figure 2). No evidence for publication bias was identified by funnel plot inspection (Supplementary Figure 3) or by the Egger’s statistical test (P=0.525). In univariate meta-regression analyses of all included studies (Table 2) the proportion of AF detection by ICM was positively associated with the duration of monitoring (coefficient=0.009; 95% CI, 0.005 to 0.013; P<0.001) (Figure 1A) and mean patient age (P=0.018) (Supplementary Figure 4A). No associations were detected with other patient characteristics (Supplementary Figures 5A and 9A), including sex (P=0.100) (Supplementary Figure 5A), hypertension (P=0.215) (Supplementary Figure 6A), diabetes mellitus (P=0.140) (Supplementary Figure 7A), mean patient CHA2DS2-VASc score (P=0.232) (Supplementary Figure 8A), or elapsed time from IS/TIA onset to cardiac monitor implantation (P=0.363) (Supplementary Figure 9A). In multivariate analyses, both monitoring duration (coefficient=0.015; 95% CI, 0.005 to 0.024; P=0.003) and mean patient age (coefficient=0.009; 95% CI, 0.003 to 0.015; P=0.004) were independently associated with the proportion of AF detection (Table 2).
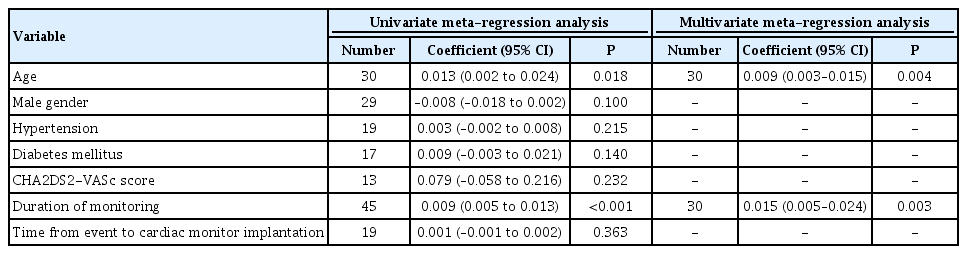
Univariate and multivariate meta-regression analyses of the association of patient and study characteristics with the percentage of patients detected with atrial fibrillation after implantable loop recorder insertion

Meta-regression analysis of the association of monitoring duration with the rate of atrial fibrillation (AF) detection with implantable cardiac monitors reported, in (A) all included (abstracts and full publications) studies and (B) fully published studies. AF incidence was calculated using the double arcsine Freeman-Tukey transformation (FTT).
In the sensitivity univariate meta-regression analyses of published studies (Supplementary Table 4) duration of ICM (coefficient=0.007; 95% CI, 0.001 to 0.014; P=0.049) (Figure 1B), history of hypertension (coefficient=0.005; 95% CI, 0.001 to 0.010; P=0.029) (Supplementary Figure 6B), and diabetes mellitus (coefficient=0.013; 95% CI, 0.001 to 0.024; P=0.033) (Supplementary Figure 7B) were positively associated with higher rates of AF detection, while no association was detected with other patient characteristics (Supplementary Figures 4B, 5B, 8B, and 9B). However, in multivariate analyses only monitoring duration (coefficient=0.009; 95% CI, 0.003 to 0.015; P=0.006) and mean patient age (coefficient=0.037; 95% CI, 0.013 to 0.062; P=0.007) were independently associated with the proportion of AF detection (Supplementary Table 4).
In subgroup analyses of all included studies (Table 3), there were significant differences (P for subgroup differences <0.001) in the rates of AF detection in subgroups stratified by ICM duration (<6 months: 5% [95% CI, 3% to 6%]; ≥6 and ≤12 months: 21% [95% CI, 16% to 25%]; >12 and ≤24 months: 26% [95% CI, 22% to 31%]; and >24 months: 34% [95% CI, 29% to 39%]) (Supplementary Figure 10). No differences were found in the subgroup analyses of all included studies stratified by study type (P=0.178) (Supplementary Figure 11), IS/TIA subgroup (P=0.093) (Supplementary Figure 12), the time threshold used for AF definition (P=0.234) (Supplementary Figure 13), the elapsed time from IS/TIA onset to cardiac monitor implantation (P >0.999) (Supplementary Figure 14) or the device used (P=0.174) (Supplementary Figure 15). Similarly, in the subgroup analysis of published studies (Supplementary Table 5) there were significant differences (P for subgroup differences <0.001) in AF detection rates in subgroups stratified by ICM duration (<6 months: 5% [95% CI, 3% to 6%]; ≥6 and ≤12 months: 22% [95% CI, 16% to 28%]; and >12 and ≤24 months: 22% [95% CI, 14% to 29%]) (Supplementary Figure 16). There were no differences in subgroup analyses of published studies stratified by IS/TIA subtype (P=0.429) (Supplementary Figure 17), the time threshold used for AF definition (P=0.149) (Supplementary Figure 18), the elapsed time from IS/TIA onset to cardiac monitor implantation (P=0.864) (Supplementary Figure 19), or the device used (P=0.174) (Supplementary Figure 20). In the aforementioned subgroup analyses, considerable heterogeneity was found within all subgroups (I2>70%), except for the subgroup of studies reporting the 6 min interval as a threshold for AF detection (I2=8%) and the subgroup of studies reporting more than 24 months of ICM duration (I2=24%).

Subgroup analyses of the association of baseline characteristics with the percentage of patients detected with atrial fibrillation after implantable loop recorder insertion
Finally, in a post hoc analysis of available studies, we found that among IS/TIA patients with AF detected during ICM, a total of 87% (95% CI, 78% to 96%) experienced asymptomatic AF episodes (Supplementary Figure 21), with no evidence of heterogeneity between studies (I2=29%, P for Cochran Q=0.21).
Discussion
Our meta-analysis showed that AF detection in patients with history of IS/TIA is positively associated only with the duration of ICM and patient age. We failed to find any other independent association between AF detection rates and IS/TIA subtype, device type, other patient characteristics, or elapsed time between IS/TIA onset and cardiac monitor implantation. Approximately nine out of 10 patients, with positive ICM for AF, experienced asymptomatic AF episodes during the monitoring period.
Our findings agree with a previously published systematic review and meta-analysis reporting improved AF detection with ICM, compared to wearable devices, in CS patients (23.3% [95% CI, 13.83% to 32.29%] vs. 13.6% [95% CI, 7.91% to 19.32%]; P for subgroup differences <0.05) [49]. However, compared to the previous meta-analysis, we included a significantly higher number of studies and patients (seven studies with 774 patients vs. 28 studies with 4,531 patients). Moreover, we assessed the potential modifying effect of stroke subtype, baseline characteristics, and time interval between ischemic event and implantation.
AF detection rates in patients with IS/TIA were unrelated to any patient characteristics, except for mean patient age. Although increased age, increased stroke severity, left atrial enlargement, hypertension, congestive heart failure, and valvular heart disease have been associated with increased incidence of AF detection in IS patients [50], proposed prediction scores including these parameters have limited diagnostic yield, especially at their middle grades [51]. Our meta-analysis also provides no further support to the theoretical concern regarding increased AF detection during the immediate post-IS period, due to stroke-induced sympathetic activation [52]. Finally, the results of subgroup analysis, regarding the time threshold used for AF definition, do not confirm the association of improved ICM performance with increased duration of AF episodes [53].
Another intriguing finding was that we observed no differences in AF detection rates using ICM, between CS and ESUS patients. This observation challenges the notion that paroxysmal AF is the main underlying etiopathogenic mechanism of cerebral ischemia in ESUS patients [54] and is in line with the recently reported New Approach Rivaroxaban Inhibition of Factor Xa in a Global Trial versus ASA to Prevent Embolism in Embolic Stroke of Undetermined Source (NAVIGATE ESUS) trial, where the detection rate of symptomatic AF during an approximate 1-year follow-up period was only 3% [55].
Several limitations of the present study need to be acknowledged. Firstly, it should be noted that baseline characteristics of individual patients (Supplementary Table 2) and study protocol parameters (Table 1) were unavailable in a significant proportion of included studies, and particularly in abstracts from conference proceedings. Moreover, the presence of ecological bias cannot be excluded; thus, the associations of aggregate patient characteristics may not hold true also for individual patient characteristics. Secondly, since this is a studylevel meta-analysis, we could not assess the influence of other parameters on AF detection rates that were not originally provided by included studies, e.g., the recently published hypertension, age, valvular heart disease, peripheral vascular disease, obesity, congestive heart failure, and coronary artery disease (HAVOC) score [49]. Thirdly, although meta-regression analyses did not provide evidence for any association bet=ween reported study characteristics (except for hypertension history) and AF detection rate, there is a possibility that heterogeneity in AF incidence could at least partially reflect inherent differences in the patient populations of included studies. Additionally, it should be highlighted that the lack of significant associations could be attributed to the low statistical power, especially for analyses including a low number of studies. Finally, it should be noted that in the present meta-analysis, we did not assess the number of AF episodes, false positive AF episodes, cumulative AF episode duration, or the impact of AF detection in patient management and long-term outcomes [56].
Our findings challenge current AHA/ASA guidelines [8,9], while further highlighting the indispensable role of prolonged rhythm monitoring, using ICM in the identification of a substantial portion of IS/TIA patients with occult AF. According to current recommendations, secondary CS prevention strategies are mainly based on antiplatelet therapy [6], which is known to provide inadequate protection for patients with AF. In these patients, the systemic administration of anticoagulant therapy could contribute to an 8.4% annual absolute risk reduction of stroke recurrence, compared with antiplatelet therapy [57]. Also taking into account the negative results of the recent NAVIGATE ESUS trial, showing that rivaroxaban compared to aspirin increases major bleeding without reducing ischemic events in ESUS patients [54], ICM emerges as an extremely useful diagnostic tool to identify those patients with occult AF within the heterogeneous group of ESUS or CS patients [58]. Therefore, prolonged monitoring could have a substantial impact on the secondary prevention of CS patients with underlying AF, leading to prompt anticoagulant initiation and lower stroke recurrence [59]. The Detection of Silent Atrial Fibrillation aFter Ischemic StrOke (SAFFO) study, guided by implantable loop recorder [60] and the AF detected by continuous electrocardiographic monitoring using implantable loop recorder to prevent stroke in individuals at risk (LOOP) study [61] are two ongoing, multicenter, open-label RCTs, that aim to evaluate health benefits, including reduction of recurrent ischemic events and cost-effectiveness of ICM in secondary stroke prevention. Results from these studies will further characterize the target population for ICM, the optimal threshold for AF definition and whether ICM monitoring results in lower stroke recurrence through anticoagulant initiation.
In conclusion, the results of the present meta-analysis support extended-duration ICM monitoring as a reasonable option for patients with IS or TIA, and initial negative screening for AF detection [62], that may substantially enhance detection of predominantly subclinical AF episodes.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2019.01067.
Supplementary Methods
Excluded studies with reasons for exclusion
Characteristics of patients in included studies
Quality assessment of included studies
Univariate and multivariate meta-regression analyses of fully published studies on the association of monitoring duration and individual patient characteristics with the rate of atrial fibrillation detection using implantable cardiac monitoring
Subgroup analyses of fully published studies on the association of monitoring duration and individual patient characteristics with the rate of atrial fibrillation detection using implantable cardiac monitors
Flow chart summarizing the selection procedure for eligible studies. ICM, implantable cardiac monitor.
Overall analysis of the cumulative rate of atrial fibrillation detection with implantable cardiac monitors. CI, confidence interval; EV/Trt, events/treated.
Funnel plot of publication bias assessment. SE, standard error; ES, effect estimate.
Meta-regression analysis of the association of mean patient age with the rate of atrial fibrillation detection with implantable cardiac monitors reported in (A) all included (abstracts and full publications) studies and (B) fully published studies. Atrial fibrillation incidence was calculated using the double arcsine Freeman-Tukey transformation.
Meta-regression analysis of the association of sex with the rate of atrial fibrillation detection with implantable cardiac monitors reported in (A) all included (abstracts and full publications) studies and (B) fully published studies. Atrial fibrillation incidence was calculated using the double arcsine Freeman-Tukey transformation.
Meta-regression analysis of the association of hypertension with the rate of atrial fibrillation detection with implantable cardiac monitors reported in (A) all included (abstracts and full publications) studies and (B) fully published studies. Atrial fibrillation incidence was calculated using the double arcsine Freeman-Tukey transformation.
Meta-regression analysis of the association of diabetes mellitus with the rate of atrial fibrillation detection with implantable cardiac monitors reported in (A) all included (abstracts and full publications) studies and (B) fully published studies. Atrial fibrillation incidence was calculated using the double arcsine Freeman-Tukey transformation.
Meta-regression analysis of the association of mean patient CHA2DS2VASc score with the rate of atrial fibrillation detection with implantable cardiac monitors reported in (A) all included (abstracts and full publications) studies and (B) fully published studies. Atrial fibrillation incidence was calculated using the double arcsine Freeman-Tukey transformation.
Meta-regression analysis on the association of mean elapsed time from ischemic stroke or transient ischemic attack onset to cardiac monitor implantation, with the rate of atrial fibrillation detection with implantable cardiac monitors reported in (A) all included (abstracts and full publications) studies and (B) fully published studies. Atrial fibrillation incidence was calculated using the double arcsine Freeman-Tukey transformation.
Subgroup analysis of the rate of atrial fibrillation detection with implantable cardiac monitors, stratified by study type (prospective or retrospective cohort), in all included studies. CI, confidence interval; EV/Trt, events/treated.
Subgroup analysis of the rate of atrial fibrillation detection with implantable cardiac monitors, stratified by monitoring duration (<6, ≥6 and ≤12, >12 and ≤24, and >24 months), in all included studies. CI, confidence interval; EV/Trt, events/treated.
Subgroup analysis of the rate of atrial fibrillation detection with implantable cardiac monitors, stratified by ischemic stroke subtype (cryptogenic stroke [CS], embolic stroke of undetermined source [ESUS], unselected ischemic stroke/transient ischemic attack), in all included studies. CI, confidence interval; EV/Trt, events/treated.
Subgroup analysis of the rate of atrial fibrillation detection with implantable cardiac monitors, stratified by the time threshold used for atrial fibrillation diagnosis (30 seconds, 2 minutes, 6 minutes), in all included studies. CI, confidence interval; EV/Trt, events/treated.
Subgroup analysis of the rate of atrial fibrillation detection with implantable cardiac monitors, stratified by the elapsed time between ischemic stroke/transient ischemic attack onset and implantation of cardiac monitor (≤1 and >1 month), in all included studies. CI, confidence interval; EV/Trt, events/treated.
Subgroup analysis of the rate of atrial fibrillation detection with implantable cardiac monitors, stratified by the type of device used, in all included studies. CI, confidence interval; EV/Trt, events/treated.
Subgroup analysis of the rate of atrial fibrillation detection with implantable cardiac monitors, stratified by monitoring duration (<6, ≥6 and ≤12, >12 and ≤24 months), in fully published studies. CI, confidence interval; EV/Trt, events/treated.
Subgroup analysis of the rate of atrial fibrillation detection with implantable cardiac monitors, stratified by ischemic stroke subtype (cryptogenic stroke [CS], embolic stroke of undetermined source [ESUS]), in fully published studies. CI, confidence interval; EV/Trt, events/treated.
Subgroup analysis of the rate of atrial fibrillation detection with implantable cardiac monitors, stratified by the time threshold used for atrial fibrillation diagnosis (30 seconds and 2 minutes) in fully published studies. CI, confidence interval; EV/Trt, events/treated; NA, not available.
Subgroup analysis of the rate of atrial fibrillation detection with implantable cardiac monitors, stratified by the elapsed time between ischemic stroke/transient ischemic attack onset and implantation of cardiac monitor (≤1 and >1 month), in fully published studies. CI, confidence interval; EV/Trt, events/treated.
Subgroup analysis of the rate of atrial fibrillation detection with implantable cardiac monitors, stratified by the type of device used, in all included studies. CI, confidence interval; EV/Trt, events/treated.
Pooled analysis of the proportion patients with episodes of asymptomatic atrial fibrillation, among patients with episodes of atrial fibrillation (both asymptomatic and symptomatic) detected with implantable cardiac monitoring. CI, confidence interval; EV/Trt, events/treated.
Supplementary References
Notes
Disclosure
The authors have no financial conflicts of interest.