Mechanical Thrombectomy for In-Hospital Onset Stroke: A Comparative Systematic Review and Meta-Analysis
Article information
Abstract
Background and Purpose
In-hospital onset stroke (IHOS) accounts for a significant proportion of large vessel occlusion acute ischemic strokes, leading to worse outcomes due to delays in evaluation and treatment. Limited data is available on the effectiveness of mechanical thrombectomy in IHOS patients. This study aims to assess the safety and efficacy of mechanical thrombectomy for patients with IHOS and compare the outcomes with those of community-onset strokes (COS).
Methods
We conducted a systematic review and meta-analysis following established guidelines, by searching PubMed, Scopus, Web of Science, and Embase databases up to April 11, 2023. Eligible studies reporting outcomes of interest were included, and relevant data was extracted and analyzed using Stata software version 17.0.
Results
In a meta-analysis of nine studies, comprising 540 cases of IHOS and 5,744 cases of COS, IHOS cases had a significantly lower rate of good functional outcomes on follow-up (35.46% vs. 40.74%, P<0.01) and a higher follow-up mortality rate (26.29% vs. 18.08%, P<0.01) compared to COS patients. Both groups had comparable successful recanalization rates (IHOS: 79.32% vs. COS: 81.44%, P=0.11), incidence rates of periprocedural complications (IHOS: 15.10%, COS: 12.96%, P=0.78), and symptomatic intracranial hemorrhage (IHOS: 6.24%, COS: 6.88%, P=0.67). It is worth noting that much of the observed effect size for mortality and good functional outcomes on follow-up was derived from only one and two studies, respectively.
Conclusion
While the current literature suggests that mechanical thrombectomy is a safe and effective treatment for IHOS, further research is necessary to comprehensively evaluate its impact, particularly during follow-up.
Introduction
In-hospital onset stroke (IHOS) is a serious medical emergency that accounts for 6.5% to 15% of all strokes. Patients with IHOS usually exhibit specific predisposing risk factors and conditions prone to stroke, such as ongoing cardiovascular disease, surgeries, or invasive procedures [1,2].
Previous studies have consistently shown that patients with IHOS experience significant delays in both evaluation and treatment, leading to worse functional outcomes compared to other stroke patients [2,3]. Despite endovascular thrombectomy being the standard acute treatment for large vessel occlusion strokes, there is limited data available on the efficacy and safety of mechanical thrombectomy in IHOS patients [3,4]. While randomized clinical trials have investigated the impact of mechanical thrombectomy on the outcomes of patients with acute ischemic stroke, these studies have primarily focused on patients who present to the emergency department from the community setting and follow specific treatment pathways for stroke. However, a notable proportion of all strokes occur in hospitalized patients, who may not be included in specific protocols for acute ischemic stroke management [3,5]. Moreover, these patients differ from the general population because they carry concurrent acute medical conditions requiring a hospital stay and are more often ineligible to receive intravenous thrombolysis [3,6]. As a result, the efficacy and safety of mechanical thrombectomy in patients with IHOS compared to community-onset stroke (COS) remain unclear. This systematic review and meta-analysis aim to clarify these points by evaluating the outcomes of mechanical thrombectomy for patients with IHOS. Specifically, we will assess the efficacy and safety of mechanical thrombectomy in IHOS patients, identifying any notable differences in treatment outcomes between IHOS and COS patients as reported in the included studies. Additionally, we will compare the workflow metrics of mechanical thrombectomy and baseline characteristics between these two groups.
Methods
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines [7], we conducted a comprehensive literature search on April 11, 2023, using the PubMed, Scopus, Web of Science, and Embase databases. We employed tailored search terms for each database, including (“in-hospital stroke” OR “in-hospital ischemic stroke” OR “in-hospital acute ischemic stroke” OR “in-hospital cerebral ischemia”) AND (“mechanical thrombectomy” OR “endovascular thrombectomy” OR “endovascular therap*” OR “endovascular treatment*” OR “clot retrieval” OR “clot disruption*” OR “clot fragmentation*” OR “stent retrieval” or “stent-assisted retrieval” OR “stentriever” OR “aspiration catheter*” OR “aspiration thrombectomy”). We also performed a manual search of references from the included studies to minimize the risk of missing relevant papers. The screening process involved a meticulous evaluation of each article’s title, abstract, and/or full text, with any uncertainties or ambiguities resolved through consultation with a senior coauthor.
All studies reporting the outcomes of mechanical thrombectomy for patients with IHOS were included in our analysis, without any restrictions on date, country of origin, or study design. We excluded duplicate papers, non-English literature, case reports, case series with less than five eligible patients, conference abstracts, editorial comments, review articles, and irrelevant papers. From each eligible study, we extracted data of interest including the first author’s name, publication year, study design, sample size, demographic and clinical characteristics of patients, workflow metrics of mechanical thrombectomy, and all variables related to the outcomes of intervention for patients with IHOS. If any, the corresponding variables for patients with COS were also collected from the included studies.
We considered achieving a modified Rankin Scale (mRS) score of 2 or lower as a good functional outcome on discharge and follow-up. Successful recanalization was defined as achieving a score of 2b or 3 on either the Thrombolysis in Cerebral Infarction (TICI) or modified TICI (mTICI) scales.
In order to evaluate the potential bias in the observational, non-randomized studies included in our analysis, we employed the Risk Of Bias in Non-randomized Studies - of Interventions (ROBINS-I) tool. This tool assesses seven distinct domains of bias, including confounding, selection of participants into the study, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes, and selection of the reported result. Our assessment yielded an overall judgment of the risk of bias, which we categorized as low, moderate, serious, or critical [8].
Statistical analysis
After extracting data, the number of subjects with and without each outcome and baseline characteristic was determined based on reported rates. Due to the methodological heterogeneity observed in the included studies, a random-effects proportion meta-analysis was carried out to pool the variable rates in the IHOS and COS groups. Confidence intervals (CIs) for these proportions were calculated using the Wilson score method [9]. Following this, we performed an odds ratio (OR) meta-analysis to compare the rates of each primary outcome or baseline characteristic between IHOS and COS cases. The forest plots reported the effect sizes as ORs using the random effects restricted maximum likelihood (REML) model.
Only temporal workflow checkpoints that were reported in at least three studies were taken into consideration for the analysis. Since the data corresponding to workflow time points displayed a skewed distribution, median values and interquartile ranges were used in most included studies for reporting purposes. Thus, the meta-analysis for the workflow findings employed the meta-analysis of the medians method, following the recommended techniques suggested by McGrath et al. [10]. In order to establish CIs for the workflow data, the quantile estimation method was employed, as it is known for its effective treatment of skewed data [10,11].
Heterogeneity was evaluated using the I2 statistic, and the Galbraith plot was used to detect outliers for each primary outcome [12]. The threshold for substantial heterogeneity was defined as an I2 value exceeding 50%, while an I2 value exceeding 75% was classified as very high heterogeneity. Moderate heterogeneity was associated with an I2 value close to 50%. Heterogeneity was considered small when the I2 value was below 50%. Furthermore, an I2 value below 10% or close to 0% indicated no to minimal heterogeneity. Furthermore, a leave-one-out analysis was conducted to verify whether the observed effect resulted from a few studies. Funnel plots were used to evaluate publication bias and were inspected visually for asymmetry. We opted not to perform the Egger’s test due to the limited number of studies included, which was fewer than ten [13,14].
Stata software (Version 17.0; StataCorp., College Station, TX, USA) was used for all statistical analyses, and the “Metapreg”user-made Stata package [15] and built-in “meta” command were utilized for the proportion and OR meta-analyses of the outcomes and baseline characteristics. Furthermore, the MetaMedian package [16] in R (version 4.2.1; R Foundation for Statistical Computing, Vienna, Austria) was employed for the meta-analysis of temporal workflow checkpoints. The obtained meta-analysis estimates from MetaMedian were then utilized to create forest plots using the Metafor package [17].
Results
Screening and selection of articles
During the systematic literature search, a total of 4,407 articles were identified using a predefined search strategy. After removing duplicate records, 4,258 papers were screened based on their title and abstract, resulting in the exclusion of 4,235 articles. The full text of the remaining 23 papers was retrieved and thoroughly reviewed. After careful consideration, 14 articles were excluded as they did not align with the aim of the study. Ultimately, nine articles that met the inclusion criteria were identified and included. The screening process and eligibility criteria were summarized following the PRISMA guidelines, and a flow diagram is presented in Figure 1.
Study and patient characteristics
The current study incorporated nine papers, comprising a total of 540 cases of IHOS and 5,744 cases of COS. The majority of the included studies had relatively small sample sizes, with less than 40 IHOS cases in all studies except for the study by Naldi et al. [3].
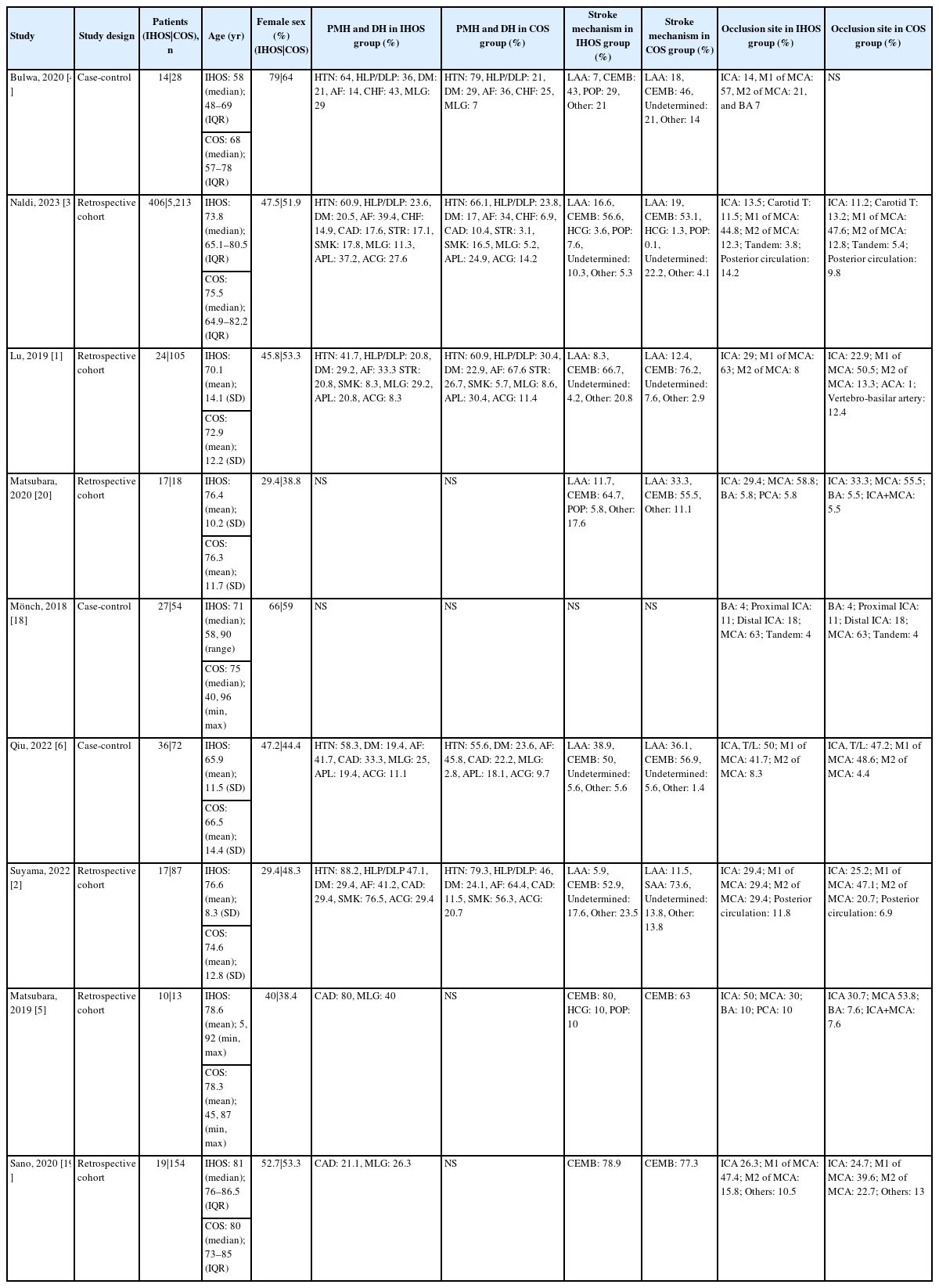
Table 1 provides an overview of the studies included and the baseline characteristics of the patients. Most of the included studies were retrospective cohort studies, while three studies had a case-control design. In both groups, cardioembolic strokes were found to be the most common mechanism of stroke. Additionally, the middle cerebral artery was the most commonly affected site of occlusion in the majority of the studies.
Table 2 includes information on procedure-related conditions and outcomes, as well as the severity of the disease as described by National Institutes of Health Stroke Scale (NIHSS) and Alberta Stroke Program Early CT Score (ASPECTS). Table 2 also includes temporal checkpoints of the procedure for both IHOS and COS groups, as reported in the studies. Most studies reported the outcomes of successful recanalization, symptomatic intracranial hemorrhage (sICH), other periprocedural complications, good outcome on discharge and follow-up, and mortality.
Quality assessment
Supplementary Table 1 provides a comprehensive summary of the results obtained from ROBINS-I tool. The table reveals that four of the studies analyzed had serious overall risk of bias concerns, while five studies had moderate concerns. In particular, confounding factors were identified as a critical issue affecting the risk of bias in all the studies. Specifically, it was expected that patients with IHOS would have more severe preexisting health problems, which could increase their likelihood of a poor outcome. While some studies controlled for various prognostic variables such as age or disease severity, none of the studies addressed the confounding effect of preexisting conditions.
Meta-analysis on procedural and treatment outcome measures
Good functional outcome
On discharge
The meta-analysis of six studies that reported relevant data revealed that the pooled rate of good functional outcomes (mRS ≤2) on discharge was 33.19% (95% CI: 17.11%–54.44%) and 33.46% (95% CI: 26.58%–41.12%) among IHOS and COS cases undergoing mechanical thrombectomy, respectively (Supplementary Figure 1A and B).
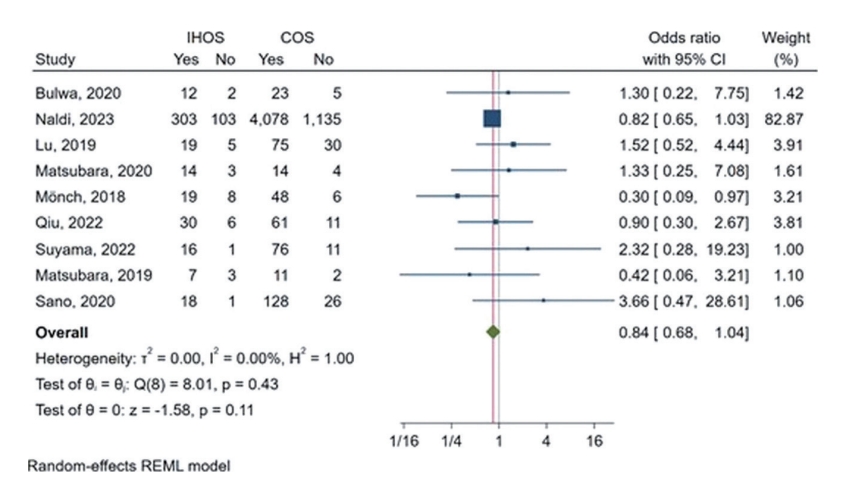
Furthermore, the comparative meta-analysis revealed no significant difference in the rate of good functional outcomes on discharge between IHOS and COS cases (pooled OR: 1.08, 95% CI: 0.57–2.05, P=0.8) (Figure 2A). Moreover, the leave-one-out analysis indicated that the observed OR was not driven by any single study, as the OR remained non-significant even when any one study was removed (Supplementary Figure 1C). The heterogeneity of the rates of this outcome in IHOS was substantial among the included studies (I2=70.19%), whereas a relatively low heterogeneity was observed among the reported rates in COS cases (I2=28.73%). The observed heterogeneity was also minimal in the OR meta-analysis (I2=24.44%). The inspection of Galbraith’s plot revealed no outlier study for this outcome.
Forest plot of the odds ratio meta-analysis for having a good functional outcome (defined as modified Rankin Scale score ≤2) at discharge (A) and follow-up (B) in mechanical thrombectomy for cases of in-hospital onset stroke (IHOS) compared to community-onset stroke (COS). CI, confidence interval; REML, restricted maximum likelihood.
On follow-up
Of the studies that reported sufficient data, five were included in the meta-analysis, which revealed a rate of 35.46% (95% CI: 31.39%–39.75%) for achieving a good functional outcome among IHOS patients (Supplementary Figure 2A) compared to the pooled rate of 40.74% (95% CI: 35.01%–46.73%) in COS patients (Supplementary Figure 2B). The duration of follow-up was typically 90 days across the included studies, although some studies did not specify a follow-up duration. A minimum to relatively low heterogeneity was observed among the reported rates (I2 <0.01% for IHOS and I2=25.60% for COS cases).
Additionally, the OR meta-analysis indicated that the rate of achieving a good functional outcome on follow-up was significantly lower among IHOS than among COS cases (OR: 0.70, 95% CI: 0.58–0.85, P<0.01) (Figure 2B). Heterogeneity was also minimal for this finding (I2<0.01%); however, our leave-one-out analysis revealed that much of the effect size was derived from only two studies (Naldi et al. [3] and Qiu et al. [6]), and removing either of these studies resulted in a non-significant observed effect (Supplementary Figure 2C). Moreover, Galbraith’s plot revealed no outlier for this outcome.
Mortality on follow-up
Five studies provided data on mortality rates during follow-up for both IHOS and COS cases. Follow-up duration was mostly 90 days, with one study being unspecified. The proportion meta-analysis revealed that IHOS cases had a pooled follow-up mortality rate of 26.29% (95% CI: 22.63%–30.32%) after undergoing mechanical thrombectomy (Supplementary Figure 3A). In comparison, the pooled rate of follow-up mortality in COS cases was 18.08% (95% CI: 17.10%–19.11%) (Supplementary Figure 3B). Additionally, OR meta-analysis showed that IHOS cases had a significantly higher chance of mortality during follow-up than COS patients (OR: 1.65, 95% CI: 1.33–2.04, P<0.01) (Figure 3). However, as with the good functional outcome rates, our leave-one-out analysis suggested that the observed effect size was largely due to one study, and excluding the study by Naldi et al. [3] would result in a non-significant effect (Supplementary Figure 3C). The observed heterogeneity for the rates and ORs of follow-up mortality was very low (I2<0.01%) with no outliers being detected.
Successful recanalization
The meta-analysis of nine studies reporting the rates of successful recanalization (TICI or mTICI 2b-3) in IHOS and COS cases found that the pooled rate of this outcome was 79.32% (95% CI: 70.36%–86.10%) and 81.44% (95% CI: 77.00%–85.19%) among IHOS and COS patients undergoing mechanical thrombectomy, respectively (Supplementary Figure 4A and 4B).
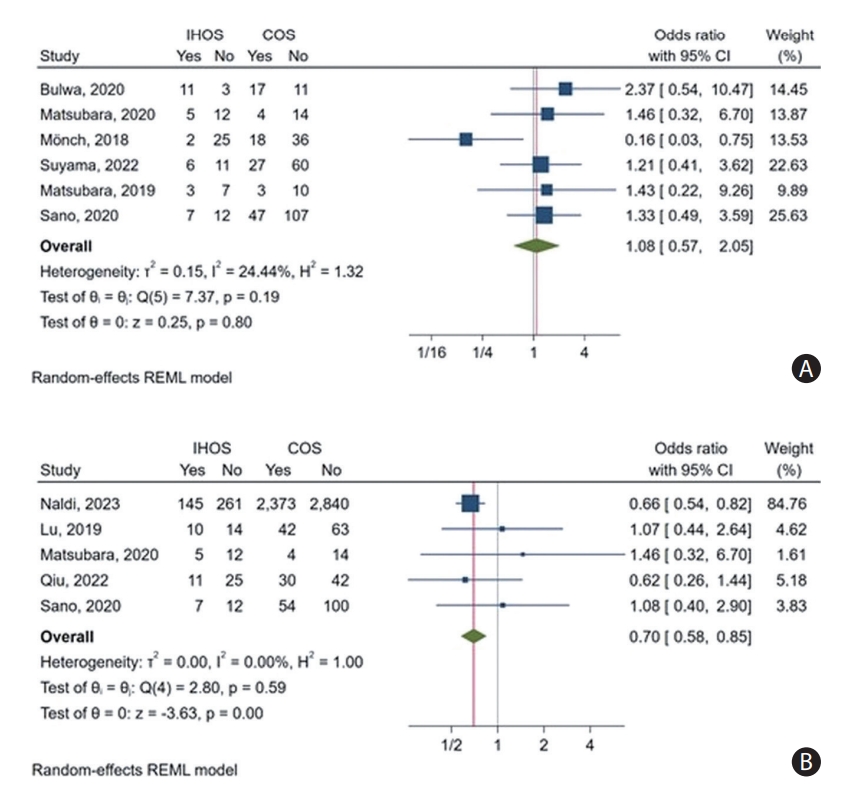
Furthermore, we conducted an OR meta-analysis and found no significant difference in the rate of successful recanalization between IHOS and COS cases (pooled OR: 0.84, 95% CI: 0.68– 1.04, P=0.11) (Figure 4). Importantly, our leave-one-out analysis indicated that these results were robust and not driven by any single study; removing any one study did not substantially affect the observed effect (Supplementary Figure 4C). The heterogeneity in this outcome across the studies included was no to small for reported rates (I2=12.38% for IHOS and I2=24.27% for COS) and ORs (I2<0.01%), and there were no outlier studies.
Periprocedural complications
Based on data from seven studies, the pooled incidence of periprocedural complications was found to be 15.10% (95% CI: 7.03%–29.50%) and 12.96% (95% CI: 6.66%–23.69%) for IHOS and COS cases, respectively (Supplementary Figure 5A and B). Our comparative meta-analysis did not reveal any significant difference in the incidence of periprocedural complications between IHOS and COS cases (OR: 1.07, 95% CI: 0.66–1.74, P=0.78) (Figure 5A). Furthermore, our leave-one-out analysis showed that the observed effect was not significantly influenced by the exclusion of any of the studies (Supplementary Figure 5C). There was moderate heterogeneity in reported rates of this outcome (I2=57.92% for IHOS and I2=67.05% for COS) and relatively low heterogeneity among the reported ORs (I2=16.87%). No outlier study was observed.
sICH
The meta-analysis of six studies reporting the rates of sICH revealed a 6.24% incidence rate among IHOS cases (95% CI: 4.47%–8.65%) and a rate of 6.88% (95% CI: 6.25%–7.57%) among COS patients (Supplementary Figure 6A and B). Additionally, the comparative meta-analysis revealed that the incidence rates of sICH did not significantly differ between IHOS and COS cases (OR: 0.92, 95% CI: 0.64–1.34, P=0.67) (Figure 5B). Our leave-one-out analysis showed that the significance of the effect was not affected by removing any individual study (Supplementary Figure 6C). The heterogeneity of this outcome among the included studies was minimal for both incidence rates and ORs (I2<0.01%), and no outlier was detected.
Meta-analysis on preprocedural intravenous thrombolytic therapy
Our meta-analysis revealed a pooled rate of intravenous tissue-type plasminogen activator (IV tPA) usage prior to mechanical thrombectomy at 15.79% (95% CI: 13.02%–19.02%) (Supplementary Figure 7A).
Additionally, IHOS cases demonstrated significantly lower IV tPA usage in comparison to COS cases (OR: 0.26, 95% CI: 0.18–0.38, P<0.01, I2=20.59%) (Supplementary Figure 7B). This significant difference was consistent across all the studies, and the omission of any individual study did not impact the statistical significance of the effect (Supplementary Figure 7C).
A publication bias analysis was conducted, revealing significant asymmetry in the funnel plot (P<0.05). However, through the trim-and-fill analysis, we imputed studies to rectify for publication bias, resulting in an OR of 0.24 (95% CI: 0.17–0.35). Remarkably, even after accounting for publication bias, the observed disparity in IV tPA usage rates between IHOS and COS cases remained statistically significant (P<0.01) (Supplementary Figure 7D).
Meta-analysis on workflow metrics
Time from onset of stroke to recognition
The meta-analysis of five studies reporting these workflow metrics did not reveal significant differences in the time intervals from the onset of stroke (last known well) to recognition (difference of medians: -27.5 minutes, 95% CI: -92.4–37.4, P=0.41, I2=84%) (Supplementary Figure 8A).
Time from stroke recognition to imaging
The meta-analysis of three studies, presenting pertinent workflow metrics, demonstrated that IHOS cases experienced a significant delay in receiving cranial imaging from the time of recognition compared to COS cases, with a median delay of 25.8 minutes (95% CI: 18.8–32.7, P<0.01, I2=48%) (Supplementary Figure 8B).
Time from onset of stroke to imaging
Utilizing data from three studies, the meta-analysis did not identify significant differences in the time intervals from the onset of stroke to imaging (difference of medians: -45.4 minutes, 95% CI: -103.5–12.7, P=0.13, I2=92.2%) (Supplementary Figure 8C).
Time from onset of stroke to groin puncture
Four studies, each reporting pertinent data, were incorporated into the analysis. Although the meta-analysis did not reveal a significant difference in the time intervals from the onset of stroke to groin puncture between the two groups (difference of medians: -24.1 minutes, 95% CI: -51.7–3.6, P=0.09, I2=9.8%), there was a non-significant trend towards a shorter time interval from the onset of stroke to groin puncture in IHOS cases (Supplementary Figure 8D).
Time from stroke recognition to groin puncture
The meta-analysis of five studies found no significant differences in the time intervals from the stroke recognition to groin puncture (difference of medians: 17.8 minutes, 95% CI: -9.8–45.3, P=0.21, I2=68%) (Supplementary Figure 8E).
Time from onset of stroke to recanalization
The meta-analysis of five studies revealed that IHOS cases achieved recanalization approximately 62.8 minutes earlier than COS cases from the time of onset of stroke (95% CI: 14.3–111.2, P=0.02, I2=65.5%) (Supplementary Figure 8F).
Time from groin puncture to recanalization
From the outcomes of the meta-analysis involving three studies, it was observed that IHOS cases achieved recanalization a significant 28.2 minutes earlier than COS cases from the time of groin puncture (95% CI: 10.7–45.7, P<0.01, I2=58.8%) (Supplementary Figure 8G).
Meta-analysis on baseline characteristics
Stroke etiology
The frequencies of cardioembolic and large artery atherosclerotic stroke etiologies among IHOS and COS cases were documented in seven and six studies, respectively. The meta-analysis indicated that there was no significant difference in the frequencies of these primary stroke etiologies between IHOS and COS cases (OR: 1.11, 95% CI: 0.92–1.34, P=0.27, I2=0% and OR: 0.83, 95% CI: 0.64–1.06, P=0.13, I2=0%, respectively) (Supplementary Figure 9).
Admission NIHSS score
The meta-analysis, which compared NIHSS scores upon admission for IHOS and COS cases, demonstrated no significant distinction in NIHSS scores between these two groups (Supplementary Figure 10).
Past medical and drug histories
OR meta-analyses were conducted to examine past medical and drug histories among IHOS and COS cases, aiming to explore potential prognosis-affecting factors.
The analysis revealed that IHOS cases exhibited higher rates of anticoagulant use and a personal history of malignancy (OR: 1.81, 95% CI: 1.11–2.97, P=0.02, I2=26% and OR: 3.78, 95% CI: 1.85–7.73, P<0.01, I2=45.26%, respectively) (Supplementary Figure 11A and B). Additionally, the prevalence of hypertension was lower in IHOS cases when compared to COS cases (OR: 0.80, 95% CI: 0.66–0.96, P=0.02, I2=0%) (Supplementary Figure 11C). However, no significant differences were observed between IHOS and COS cases in the rates of history of atrial fibrillation, diabetes mellitus, and hyperlipidemia/dyslipidemia (Supplementary Figure 11D-F).
Publication bias
The funnel plots of the publication bias are depicted in Supplementary Figure 12. The visual inspection of these plots revealed no asymmetry for most primary outcomes.
Discussion
The current systematic review and meta-analysis revealed that mechanical thrombectomy was a safe and effective treatment for IHOS patients. The procedure demonstrated a high successful recanalization rate and a comparable incidence of periprocedural complications and sICH, as well as good outcomes at discharge, when compared to cases of COS.
Mechanical thrombectomy is a promising treatment option for IHOS patients, facilitated by the availability of stroke neurology and neurointerventional teams for inpatients [1]. The limitations in using intravenous thrombolysis due to frequent contraindications from concurrent acute illnesses and baseline comorbidities in hospitalized patients with acute ischemic stroke [3,4,6] emphasize the importance of mechanical thrombectomy as a viable option for reperfusion in appropriate candidates. In a similar vein, our observation indicated that the use of IV tPA was notably less frequent in IHOS cases compared to COS cases, and this pattern was consistent across all studies.
Several in-hospital cohorts have reported successful reperfusion grades following mechanical thrombectomy, indicating the technical feasibility of this procedure for IHOS patients [1-6,18-20]. Our analysis further confirmed the high rate of successful recanalization for mechanical thrombectomy in IHOS cases, which was found to be comparable to that in COS cases.
Mechanical thrombectomy has also been established as a safe procedure for patients with IHOS in terms of sICH and other procedure-related complications, even in critically ill patients with a higher antithrombotic therapy burden [1,3,18]. Several studies have reported that the incidence of periprocedural complications was not significantly higher in IHOS patients compared to patients with COS, and the occurrence of sICHs was evenly distributed between the two groups [1-5,18-20]. Our meta-analysis also verified these results.
Additionally, we found a comparable rate of good outcomes on discharge for IHOS cases to COS patients, but a significantly lower rate of good outcomes and a higher rate of mortality on follow-up. This discrepancy may be explained by several factors, including the higher burden of acute and chronic illnesses and comorbidities in IHOS, which can negatively impact long-term recovery. Moreover, longer hospital stays can increase the risk of hospital-acquired infections and other complications, further delaying recovery and leading to worse outcomes on follow-up.
Delay in stroke symptom recognition, neurological evaluation, and appropriate intervention, reported by some studies [2,3], may also play a role, which might be related to the complexity of patients’ underlying illness and hospital practices for IHOS. In our study, while the timing of workflow checkpoints in IHOS patients was generally comparable to or slightly better than those in COS patients, the included papers reported significant delays in recognition, imaging, groin puncture, and recanalization among IHOS patients, highlighting the need for optimization. Also, it is crucial to address delays in cranial imaging for IHOS cases to ensure swift diagnosis and intervention. Ideally, workflow times should be significantly faster for IHOS patients, considering their hospitalization. Given the high frequency of unfavorable outcomes in IHOS patients [21-23], enhancing the efficiency of in-hospital stroke workflows becomes a top priority. This involves streamlining stroke team notification and emergency imaging for expedited IHOS diagnosis. Standardized guidelines for IHOS management aligning with COS time metrics are essential for consistent and effective care. Incorporating cost-effective technologies like portable imaging devices could aid in early stroke detection, especially in high-risk wards [23]. Moreover, quality improvement initiatives for IHOS protocols have proven effective in reducing time delays without compromising patient safety [22], likely benefiting the functional outcomes of mechanically thrombectomized IHOS patients.
Our study has provided valuable insights into the effectiveness of mechanical thrombectomy in IHOS patients. However, several limitations must be acknowledged. Firstly, the majority of studies included in our review had small sample sizes. This limitation may restrict the generalizability of the findings and the statistical power of the study. Secondly, the effect sizes extracted from the study by Naldi et al. [3] may significantly influence the pooled findings. Moreover, it is worth noting that the definitions of periprocedural complications were not consistent across all the studies, resulting in significant heterogeneity in the reported rates. Furthermore, none of the included studies addressed the confounding effect of preexisting medical conditions. Therefore, it is crucial to interpret the results of our review while keeping in mind this factor, which may influence the occurrence of complications and the efficacy of the treatment analyzed. Additionally, our study did not include a control group of patients who did not undergo mechanical thrombectomy, which could limit the ability to draw conclusions about the effectiveness of the intervention compared to other treatment options. Finally, the current study only focused on primary outcomes related to the effectiveness of mechanical thrombectomy in IHOS cases and the studies included in our analysis did not report long-term outcomes.
Conclusions
Our study showed remarkably high rates of successful recanalization, as well as comparable incidence of periprocedural complications and good outcome on discharge after mechanical thrombectomy in IHOS, in comparison to COS cases. However, IHOS patients had a lower good outcome and a higher mortality rate during follow-up, with the effect size primarily from a few studies, requiring further research for confirmation and expansion of the findings. Moreover, delays in receiving cranial imaging from the time of recognition were observed for IHOS cases, while achieving earlier recanalization from both stroke onset and groin puncture. Accordingly, future research should prioritize assessing the impact of time delays on patient outcomes. We would also recommend conducting more studies to assess the long-term outcomes of mechanical thrombectomy for IHOS patients. This would help to provide more comprehensive information on the efficacy and safety of this treatment for IHOS.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2023.01613.
Risk of bias assessment of included studies
Meta-analysis on good functional outcome at discharge. (A) Forest plot of the proportion meta-analysis of the rate of having a good functional outcome (defined as modified Rankin Scale score ≤2) at discharge in mechanical thrombectomy for cases of in-hospital onset stroke. (B) Forest plot of the proportion meta-analysis of the rate of having a good functional outcome (defined as modified Rankin Scale score ≤2) at discharge in mechanical thrombectomy for cases of community-onset stroke. (C) Forest plot of the leave-one-out analysis of the odds ratio meta-analysis of good functional outcome (defined as modified Rankin Scale score ≤2) at discharge after mechanical thrombectomy in cases of in-hospital onset stroke compared to community-onset stroke. CI, confidence interval; REML, restricted maximum likelihood.
Meta-analysis on good functional outcome on follow-up. (A) Forest plot of the proportion meta-analysis of the rate of having good functional outcome (defined as modified Rankin Scale score ≤2) on follow-up in mechanical thrombectomy in cases of in-hospital onset stroke. (B) Forest plot of the proportion meta-analysis of the rate of having good functional outcome (defined as modified Rankin Scale score ≤2) on follow-up in mechanical thrombectomy in cases of community-onset stroke. (C) Forest plot of the leave-one-out analysis of the odds ratio meta-analysis of good functional outcomes (defined as modified Rankin Scale score ≤2) on follow-up for mechanical thrombectomy in cases of in-hospital onset stroke compared to communityonset stroke. CI, confidence interval; REML, restricted maximum likelihood.
Meta-analysis on mortality during follow-up. (A) Forest plot of the proportion meta-analysis of the mortality rate on follow-up in mechanical thrombectomy for in-hospital onset stroke cases. (B) Forest plot of the proportion meta-analysis of the mortality rate on follow-up in mechanical thrombectomy for community-onset stroke cases. (C) Forest plot of the leave-one-out analysis of odds ratio meta-analysis for mortality on follow-up in mechanical thrombectomy for in-hospital onset stroke compared to community-onset stroke. CI, confidence interval; REML, restricted maximum likelihood.
Meta-analysis on successful recanalization. (A) Forest plot of the proportion meta-analysis for the successful recanalization rate of mechanical thrombectomy in cases of In-hospital onset stroke. (B) Forest plot of the proportion meta-analysis for the successful recanalization rate of mechanical thrombectomy in cases of community-onset stroke. (C) Forest plot of the leave-one-out analysis of odds ratio meta-analysis on the successful recanalization rate of mechanical thrombectomy in cases of in-hospital onset stroke compared to community-onset stroke. CI, confidence interval; REML, restricted maximum likelihood.
Meta-analysis on periprocedural complications. (A) Forest plot of the proportion meta-analysis for the rate of periprocedural complications of mechanical thrombectomy in cases of in-hospital onset stroke. (B) Forest plot of the proportion meta-analysis for the rate of periprocedural complications of mechanical thrombectomy in cases of community-onset stroke. (C) Forest plot of the leave-one-out analysis of odds ratio meta-analysis of periprocedural complications of mechanical thrombectomy in cases of in-hospital onset stroke compared to community-onset stroke. CI, confidence interval; REML, restricted maximum likelihood.
Meta-analysis on symptomatic intracranial hemorrhage. (A) Forest plot of the proportion meta-analysis on the rate of developing symptomatic intracranial hemorrhage as a complication of mechanical thrombectomy in cases of in-hospital onset stroke. (B) Forest plot of the proportion meta-analysis on the rate of developing symptomatic intracranial hemorrhage as a complication of mechanical thrombectomy in cases of community-onset stroke. (C) Forest plot of the leave-one-out analysis of odds ratio meta-analysis for the development of symptomatic intracranial hemorrhage as a complication of mechanical thrombectomy in cases of in-hospital onset stroke compared to community-onset stroke. CI, confidence interval; REML, restricted maximum likelihood.
Meta-analysis on preprocedural intravenous thrombolytic therapy. (A) Forest plot of the proportion meta-analysis of intravenous thrombolytic use among mechanical thrombectomy cases. (B) Forest plot of the odds ratio meta-analysis of intravenous thrombolytic use among cases of inhospital onset stroke (IHOS) compared to community-onset stroke (COS). (C) Forest plot of the leave-one-out analysis of odds ratio meta-analysis for intravenous thrombolytic use among cases of IHOS compared to COS. (D) Funnel plot for assessing publication bias of odds ratio meta-analysis for intravenous thrombolytic use among IHOS cases compared to COS cases. CI, confidence interval; REML, restricted maximum likelihood.
Meta-analysis on workflow metrics. (A) Random effects meta-analysis of the difference of median times from onset of stroke to recognition in in-hospital onset stroke cases compared to community-onset stroke cases. The effect sizes correspond to the median difference of median times from onset to recognition (in minutes). Negative values correspond to evidence of earlier recognition in in-hospital onset stroke cases. (B) Random effects meta-analysis of the difference of median times from stroke recognition to cranial imaging in in-hospital onset stroke cases compared to communityonset stroke cases. The effect sizes correspond to the median difference of median times from recognition to cranial imaging (in minutes). (C) Random effects meta-analysis of the difference of median times from onset of stroke to cranial imaging in in-hospital onset stroke cases compared to community-onset stroke cases. The effect sizes correspond to the median difference of median times from onset to imaging (in minutes). Negative values correspond to evidence of earlier imaging in in-hospital onset stroke cases. (D) Random effects meta-analysis of the difference of median times from onset of stroke to groin puncture in in-hospital onset stroke cases compared to community-onset stroke cases. The effect sizes correspond to the median difference of median times from onset to groin puncture (in minutes). Negative values correspond to evidence of earlier puncture in in-hospital onset stroke cases. (E) Random effects metaanalysis of the difference of median times from stroke recognition to groin puncture in in-hospital onset stroke cases compared to community-onset stroke cases. The effect sizes correspond to the median difference of median times from recognition to groin puncture (in minutes). Negative values correspond to evidence of earlier puncture in in-hospital onset stroke cases. (F) Random effects meta-analysis of the difference of median times from onset of stroke to recanalization in in-hospital onset stroke cases compared to community-onset stroke cases. The effect sizes correspond to the median difference of median times from onset to recanalization (in minutes). Negative values correspond to evidence of earlier recanalization in in-hospital onset stroke cases. (G) Random effects meta-analysis of the difference of median times from groin puncture to recanalization in in-hospital onset stroke cases compared to community-onset stroke cases. The effect sizes correspond to the median difference of median times from groin puncture to recanalization (in minutes). Negative values correspond to evidence of earlier recanalization in in-hospital onset stroke cases. CI, confidence interval.
Meta-analysis on stroke etiology. (A) Forest plot of odds ratio meta-analysis for cardioembolic mechanism of stroke among in-hospital onset stroke (IHOS) cases compared to community-onset stroke (COS) cases. (B) Forest plot of odds ratio meta-analysis for large artery atherosclerotic mechanism of stroke among IHOS cases compared to COS cases. CI, confidence interval; REML, restricted maximum likelihood.
Forest plot of the meta-analysis comparing National Institutes of Health Stroke Scale on admission in in-hospital onset stroke (IHOS) cases compared to community-onset stroke (COS) cases. CI, confidence interval; SD, standard deviation; REML, restricted maximum likelihood.
Meta-analysis on past medical and drug histories. (A) Forest plot of odds ratio meta-analysis for anticoagulant medication use among in-hospital onset stroke (IHOS) cases compared to community onset stroke (COS) cases. (B) Forest plot of odds ratio meta-analysis for having a personal history of malignancy among in-hospital onset stroke (IHOS) cases compared to COS cases. (C) Forest plot of odds ratio meta-analysis of having a history of hypertension among in-hospital onset stroke (IHOS) cases compared to COS cases. (D) Forest plot of odds ratio meta-analysis of having a history of atrial fibrillation among IHOS cases compared to COS cases. (E) Forest plot of odds ratio meta-analysis of having a history of diabetes mellitus among IHOS cases compared to COS cases. (F) Forest plot of odds ratio meta-analysis of having a history of hyperlipidemia/dyslipidemia among IHOS cases compared to COS cases. CI, confidence interval; REML, restricted maximum likelihood.
Publication bias analysis. (A) Funnel plot to assess publication bias of the odds ratio meta-analysis for having good functional outcome (defined as modified Rankin Scale score ≤2) on discharge after mechanical thrombectomy in cases of in-hospital onset stroke compared to community-onset stroke. (B) Funnel plot to assess publication bias of the odds ratio meta-analysis for having good functional outcome (defined as modified Rankin Scale score ≤2) on follow-up after mechanical thrombectomy in cases of in-hospital onset stroke compared to community-onset stroke. (C) Funnel plot to assess publication bias of the odds ratio meta-analysis of mortality on follow-up after mechanical thrombectomy in cases of in-hospital onset stroke compared to community-onset stroke. (D) Funnel plot to assess publication bias of the odds ratio meta-analysis for successful recanalization rate of mechanical thrombectomy in cases of in-hospital onset stroke compared to community-onset stroke. (E) Funnel plot to assess publication bias of the odds ratio meta-analysis for periprocedural complications of mechanical thrombectomy in cases of in-hospital onset stroke compared to community-onset stroke. (F) Funnel plot to assess publication bias of the odds ratio meta-analysis for developing symptomatic intracranial hemorrhage as a complication of mechanical thrombectomy in cases of in-hospital onset stroke compared to community-onset stroke. CI, confidence interval; REML, restricted maximum likelihood.
Notes
Funding statement
None
Conflicts of interest
DFK holds equity in Nested Knowledge, Superior Medical Editors, and Conway Medical, Marblehead Medical; a consultant for MicroVention, Medtronic, Balt, and Insera Therapeutics; Data Safety Monitoring Board for Vesalio; and receiving royalties from Medtronic. RK is contracted/consultant for Cerenovus Inc, Medtronic, Endovascular Engineering, Frontior Bio, Sensome Inc, Endomimetics, Ancure LLC, Neurogami Medical, MIVI Biosciences, Monarch Biosciences, Stryker Inc, Conway Medical, Pireus Medical, and Bionau Labs. All remaining authors have declared no conflicts of interest.
Author contribution
Conceptualization: SG, RK, DFK. Study design: SG, MA, AH. Methodology: AH, PV, PJ. Data collection: MA, AH, CB. Investigation: MA, AH, CB. Statistical analysis: PV, PJ, SG. Writing—original draft: MA, AH, PV, PJ. Writing—review & editing: AH, SG, CB. Funding acquisition: NA. Approval of final manuscript: all authors.
Acknowledgements
We express our gratitude to the Nested Knowledge developers, namely Karl Holub, Stephen Mead, Jeff Johnson, and Darian Lehmann-Plantenberg, whose contributions were instrumental in facilitating this study through the development of the AutoLit and Synthesis platforms for systematic review. Additionally, we would like to acknowledge the utilization of ChatGPT, an OpenAI language model based on the GPT-3.5 architecture, for providing assistance in language corrections during the manuscript editing process, thereby enhancing readability and language quality. Nevertheless, the authors take full responsibility for the publication’s content as they thoroughly reviewed and edited the material following the utilization of the tool.